Comparative Animal Physiology Osmoregulation in fishes Freshwater fish



![External Osmolarity Internal [Na+] Internal [Urea] Internal Osmolarity External Osmolarity Internal [Na+] Internal [Urea] Internal Osmolarity](https://slidetodoc.com/presentation_image_h2/5e4203774eea3406f4eeb1e8f4c92fa1/image-13.jpg)
![Internal [Na+] Internal [Urea] Internal Osmolarity Ureo-osmoconformer External Osmolarity Internal [Na+] Internal [Urea] Internal Osmolarity Ureo-osmoconformer External Osmolarity](https://slidetodoc.com/presentation_image_h2/5e4203774eea3406f4eeb1e8f4c92fa1/image-15.jpg)







- Slides: 37

Comparative Animal Physiology Osmoregulation in fishes

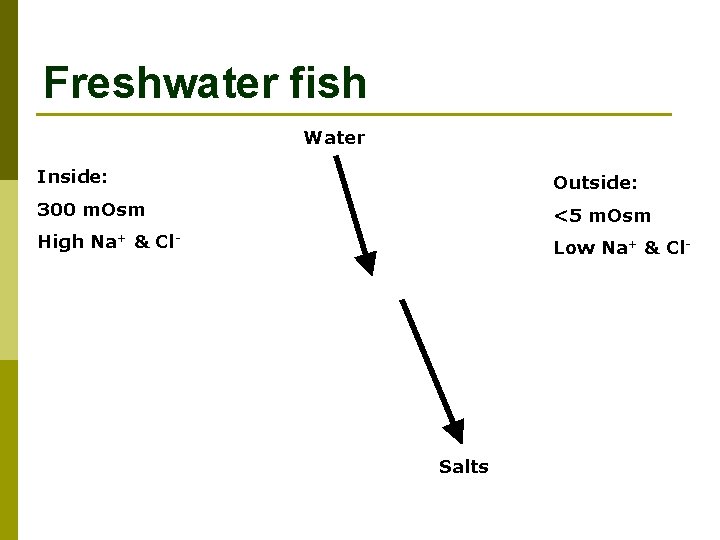
Freshwater fish Water Inside: Outside: 300 m. Osm <5 m. Osm High Na+ & Cl- Low Na+ & Cl- Salts

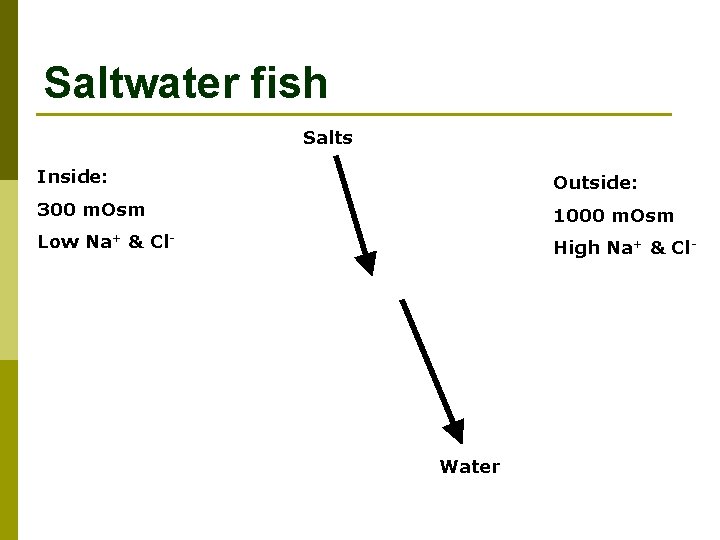
Saltwater fish Salts Inside: Outside: 300 m. Osm 1000 m. Osm Low Na+ & Cl- High Na+ & Cl- Water

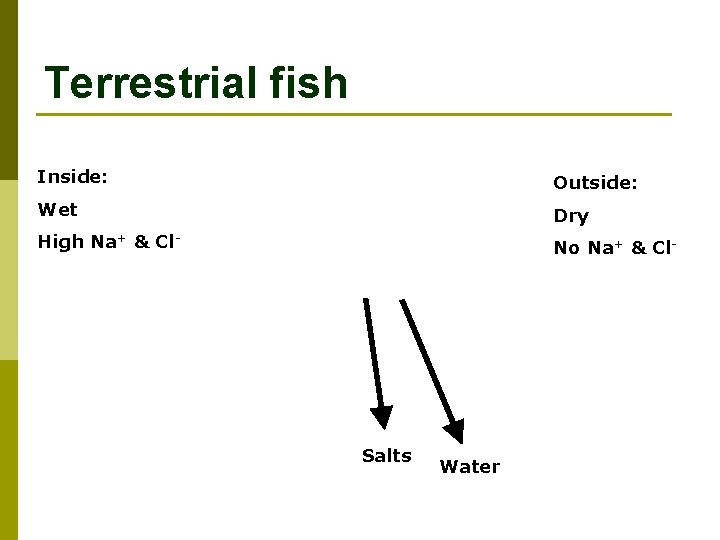
Terrestrial fish Inside: Outside: Wet Dry High Na+ & Cl- No Na+ & Cl- Salts Water

Osmoregulation p Maintenance of water and salt balance in the body p Why freshwater fishes don’t explode, saltwater fishes don’t dry up and people don’t desiccate

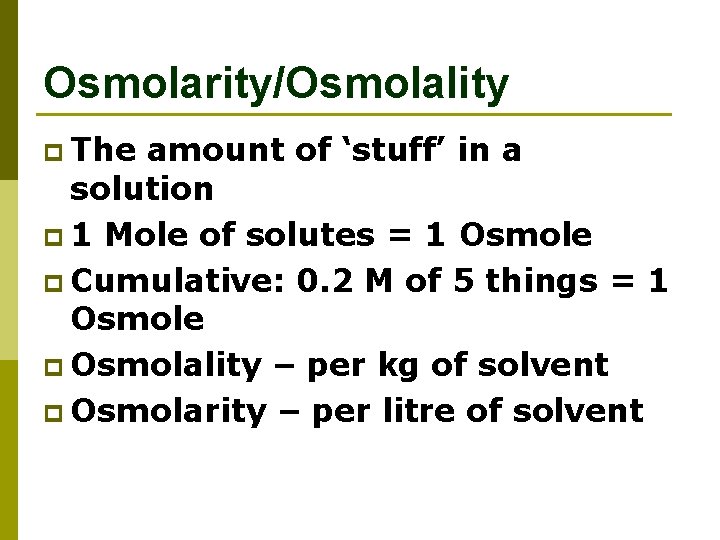
Osmolarity/Osmolality p The amount of ‘stuff’ in a solution p 1 Mole of solutes = 1 Osmole p Cumulative: 0. 2 M of 5 things = 1 Osmole p Osmolality – per kg of solvent p Osmolarity – per litre of solvent

Osmotic pressure p Solutes exert pressure that moves water from place to place p Can be a source of hydrostatic pressure…

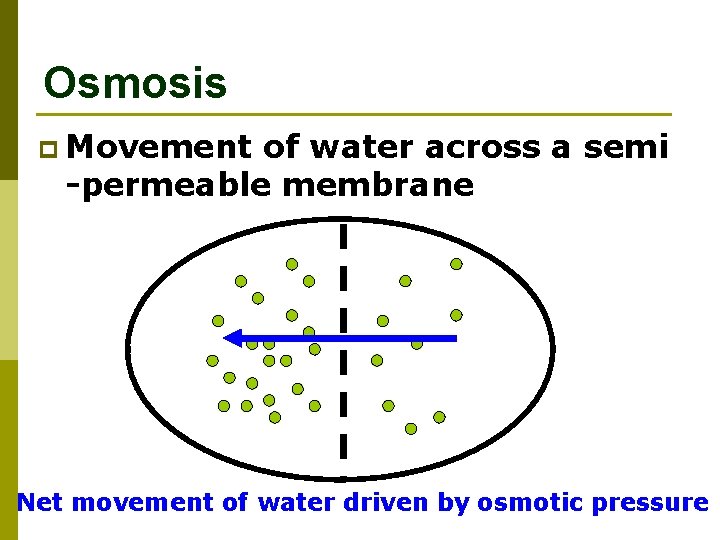
Osmosis p Movement of water across a semi -permeable membrane Net movement of water driven by osmotic pressure

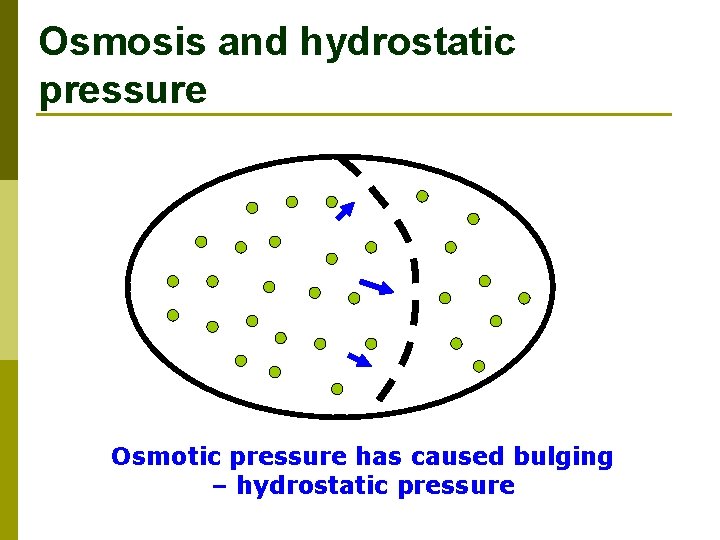
Osmosis and hydrostatic pressure Osmotic pressure has caused bulging – hydrostatic pressure

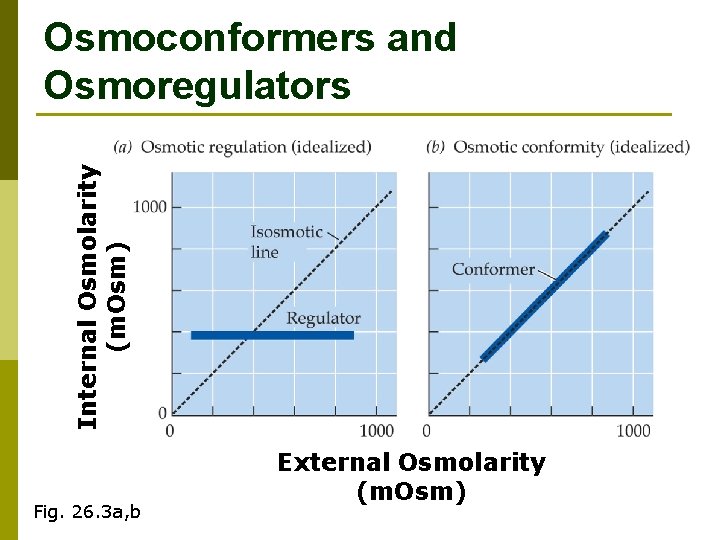
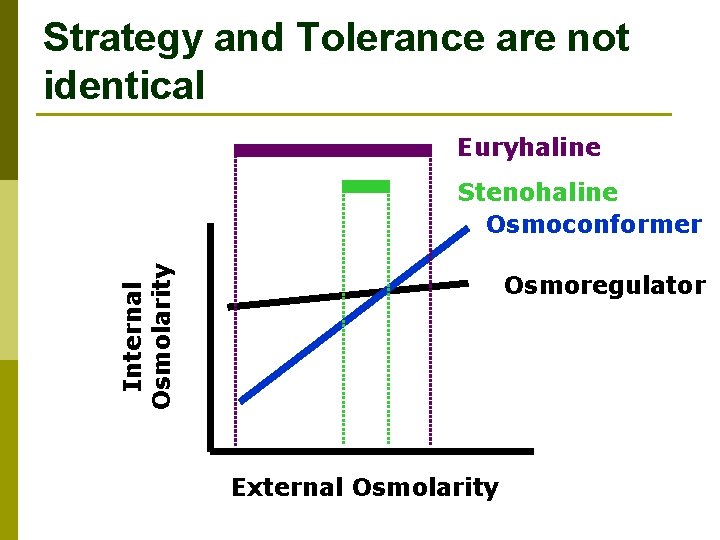
Internal Osmolarity (m. Osm) Osmoconformers and Osmoregulators Fig. 26. 3 a, b External Osmolarity (m. Osm)

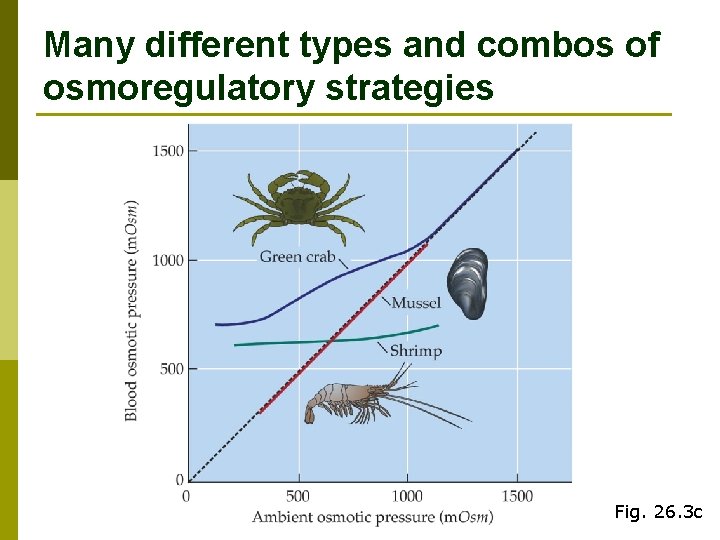
Many different types and combos of osmoregulatory strategies Fig. 26. 3 c

Strategy and Tolerance are not identical Euryhaline Internal Osmolarity Stenohaline Osmoconformer Osmoregulator External Osmolarity
![External Osmolarity Internal Na Internal Urea Internal Osmolarity External Osmolarity Internal [Na+] Internal [Urea] Internal Osmolarity](https://slidetodoc.com/presentation_image_h2/5e4203774eea3406f4eeb1e8f4c92fa1/image-13.jpg)
External Osmolarity Internal [Na+] Internal [Urea] Internal Osmolarity

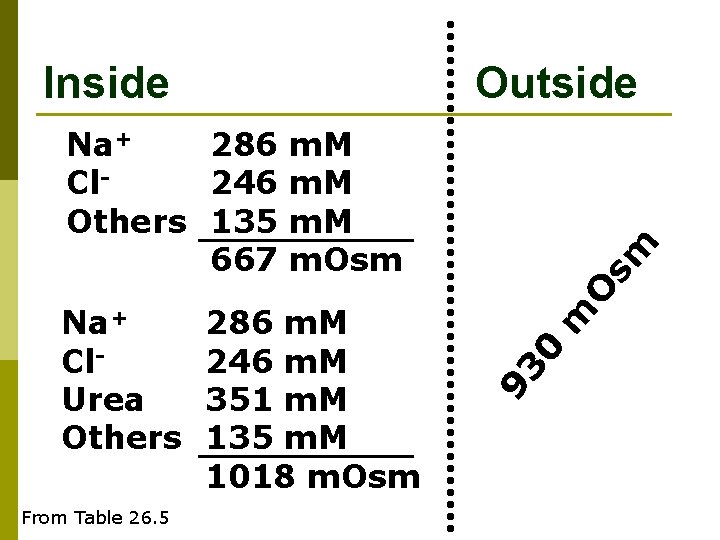
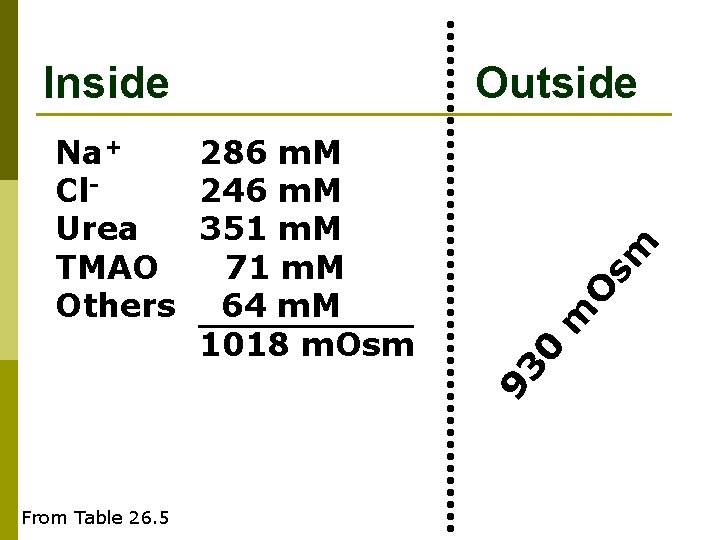
Inside Outside From Table 26. 5 m 0 286 m. M 246 m. M 351 m. M 135 m. M 1018 m. Osm 93 Na+ Cl. Urea Others O sm Na+ 286 m. M Cl 246 m. M Others 135 m. M 667 m. Osm
![Internal Na Internal Urea Internal Osmolarity Ureoosmoconformer External Osmolarity Internal [Na+] Internal [Urea] Internal Osmolarity Ureo-osmoconformer External Osmolarity](https://slidetodoc.com/presentation_image_h2/5e4203774eea3406f4eeb1e8f4c92fa1/image-15.jpg)
Internal [Na+] Internal [Urea] Internal Osmolarity Ureo-osmoconformer External Osmolarity

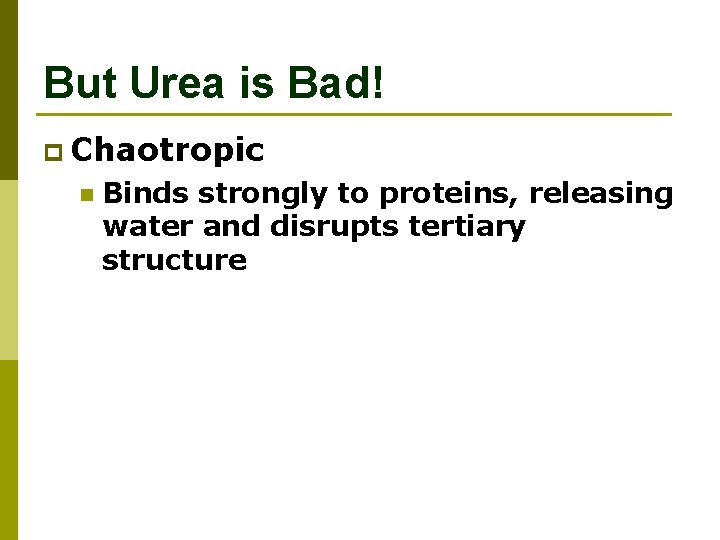
But Urea is Bad! p Chaotropic n Binds strongly to proteins, releasing water and disrupts tertiary structure

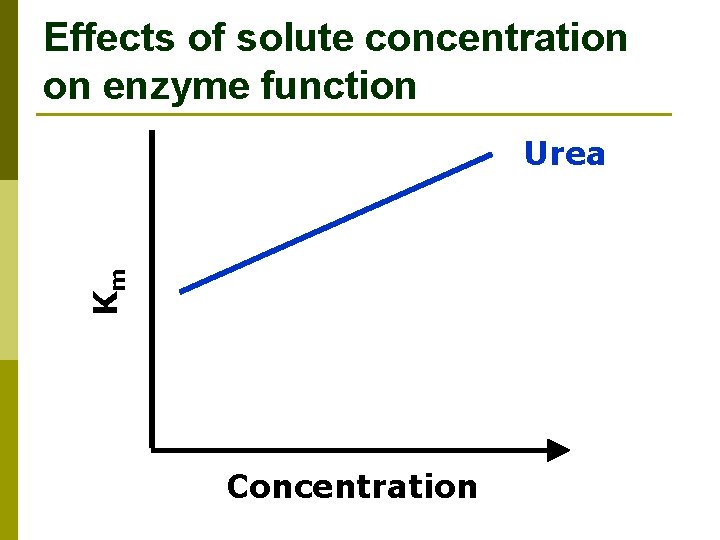
Effects of solute concentration on enzyme function Km Urea Concentration

Trimethylamine oxide (TMAO) CH 3 C N+ O- CH 3

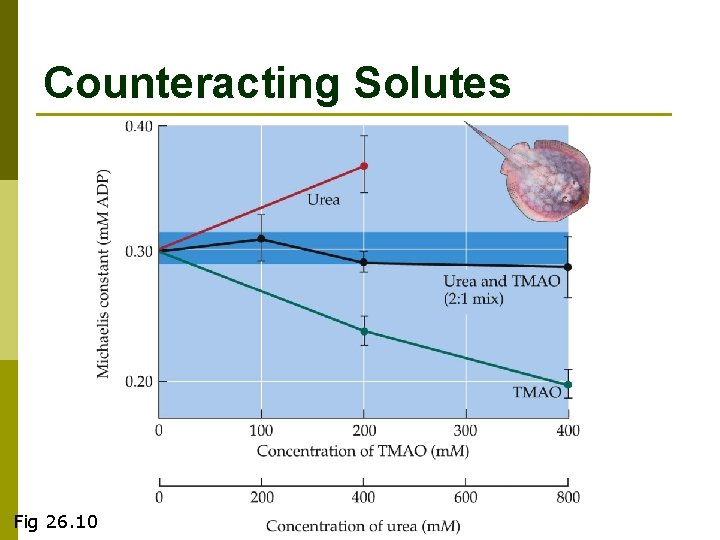
Counteracting Solutes Fig 26. 10

O sm m 93 Na+ 286 m. M Cl 246 m. M Urea 351 m. M TMAO 71 m. M Others 64 m. M 1018 m. Osm Outside 0 Inside From Table 26. 5

Ureo-Osmoconformation in sharks p Urea is used to make up the ‘osmotic gap’ between internal and external concentration n Requires high protein diet for manufacturing Urea TMAO acts as a counteracting solute to preserve protein function in high concentrations of urea. p Why would you soak shark prior to cooking it? p

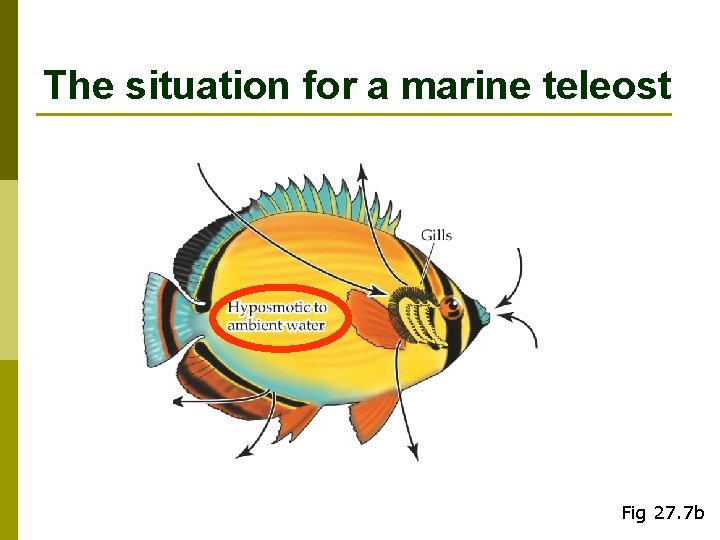
The situation for a marine teleost Fig 27. 7 b

Gills as exchange organs p CO 2 & O 2 p Used to remove the salts that are ingested with food and water (and absorbed through gill surfaces) n Major site for this in marine teleosts n

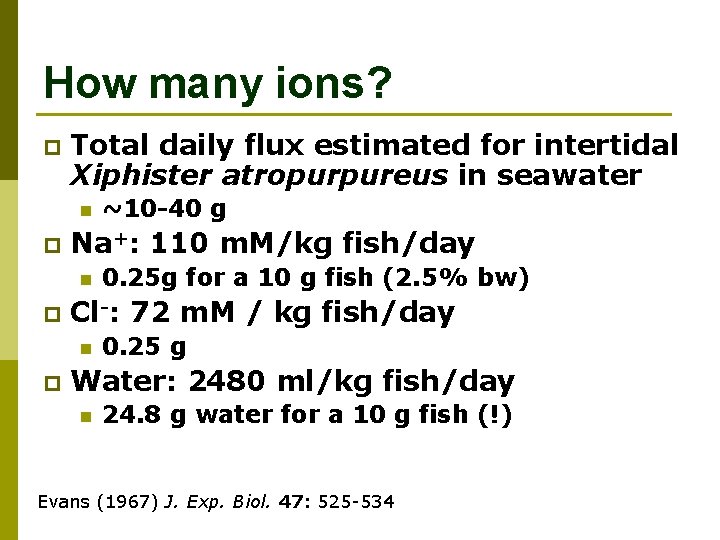
How many ions? p Total daily flux estimated for intertidal Xiphister atropurpureus in seawater n p Na+: 110 m. M/kg fish/day n p 0. 25 g for a 10 g fish (2. 5% bw) Cl-: 72 m. M / kg fish/day n p ~10 -40 g 0. 25 g Water: 2480 ml/kg fish/day n 24. 8 g water for a 10 g fish (!) Evans (1967) J. Exp. Biol. 47: 525 -534

Chloride cells Water Apical (Mucosa) Pavement cell Blood Baso-lateral (serosa) Fig. 27. 6

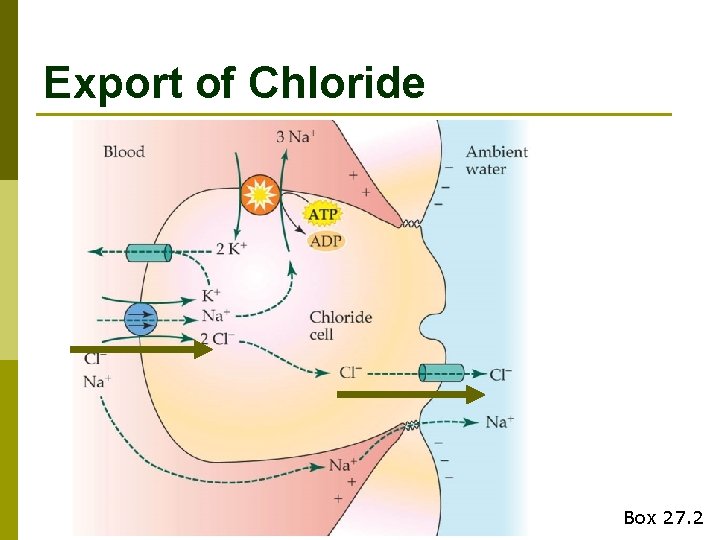
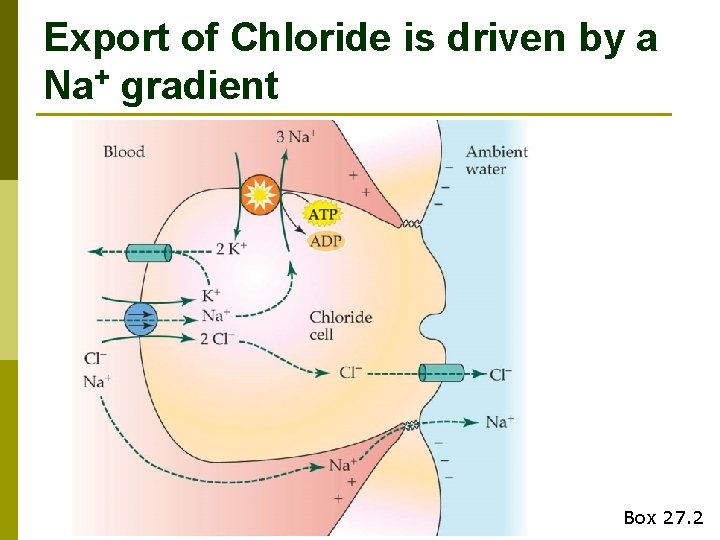
Export of Chloride Box 27. 2

Export of Chloride is driven by a Na+ gradient Box 27. 2

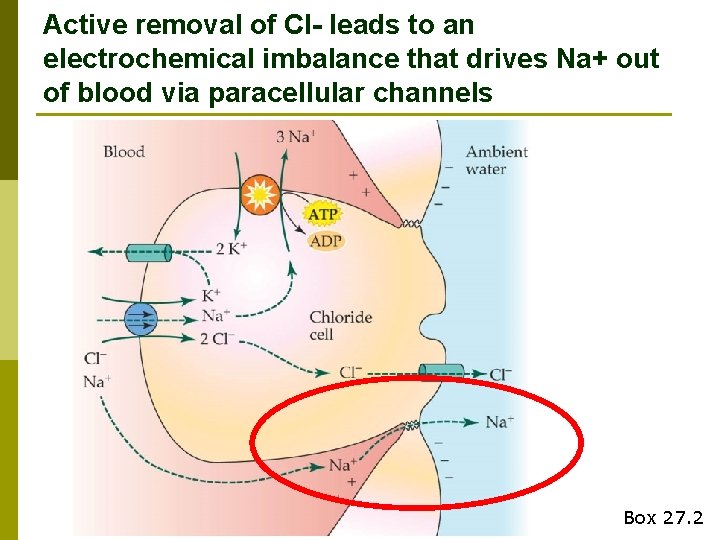
Active removal of Cl- leads to an electrochemical imbalance that drives Na+ out of blood via paracellular channels Box 27. 2

Chloride cell summary p Transcellular n transport of Cl- Driven by Na+, K+-ATPase (requires energy) p Paracellular transport of Na+ p Ionoregulation accounts for ~35% of resting MR in marine teleosts

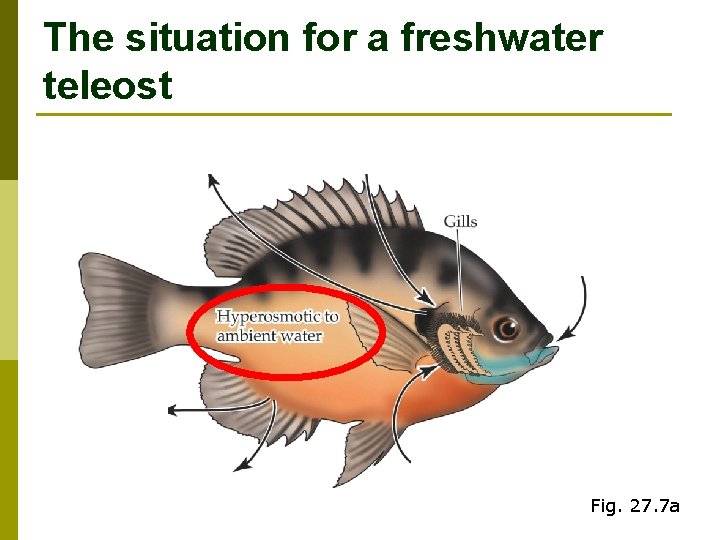
The situation for a freshwater teleost Fig. 27. 7 a

Gills as exchange organs p CO 2 & O 2 p Used to take up salts from the environment n Not much Na. Cl in freshwater, but gills process a huge volume

Chloride cells again Figs 27. 3 & 27. 4

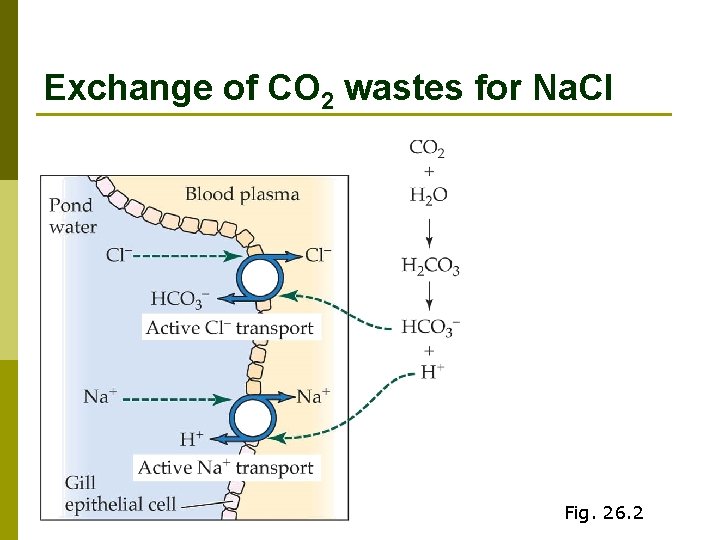
Exchange of CO 2 wastes for Na. Cl Fig. 26. 2

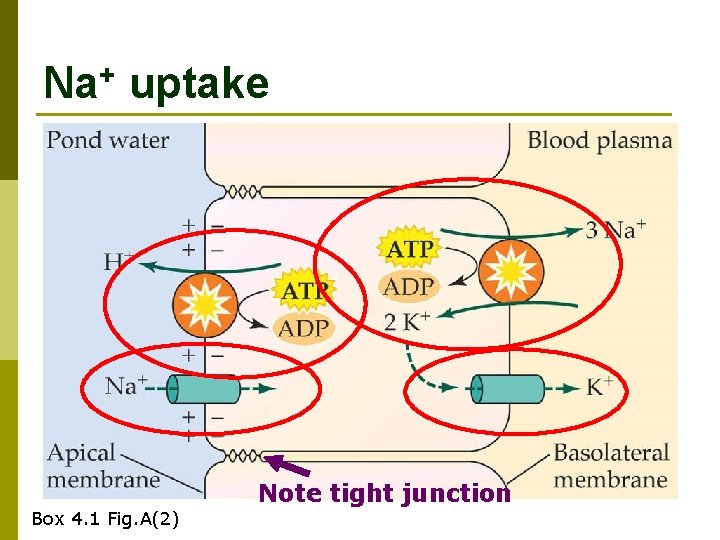
Na+ uptake Box 4. 1 Fig. A(2) Note tight junction

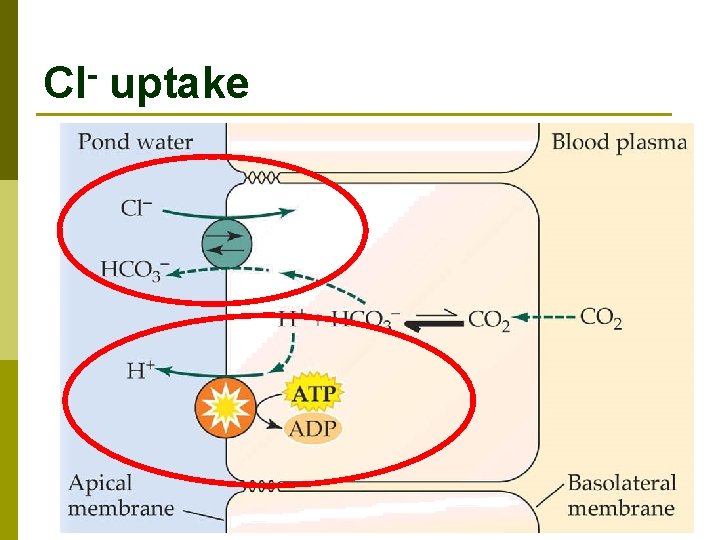
Cl- uptake

Na. Cl uptake summary p Exchange for CO 2 Na+ via electrochemical gradient n Cl- via HCO 3 - antiport n p Very dilute urine gets rid of excess water without losing too much salt

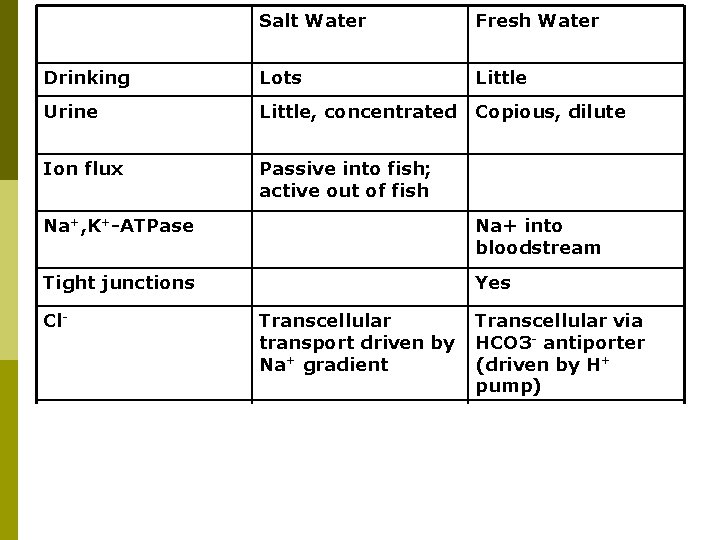
Salt Water Fresh Water Drinking Lots Little Urine Little, concentrated Copious, dilute Ion flux Passive into fish; active out of fish Na+, K+-ATPase Na+ into bloodstream Tight junctions Yes Cl- Transcellular transport driven by Na+ gradient Transcellular via HCO 3 - antiporter (driven by H+ pump) Na+ Paracellular driven by electochemical gradient Transcellular driven by electrochemical gradient (set up by H+ pump and Na+, K+-ATPase)