COMMON TECHNICAL DOCUMENT 1 19 ORIGIN OF CTD









- Slides: 19

COMMON TECHNICAL DOCUMENT 1 / 19

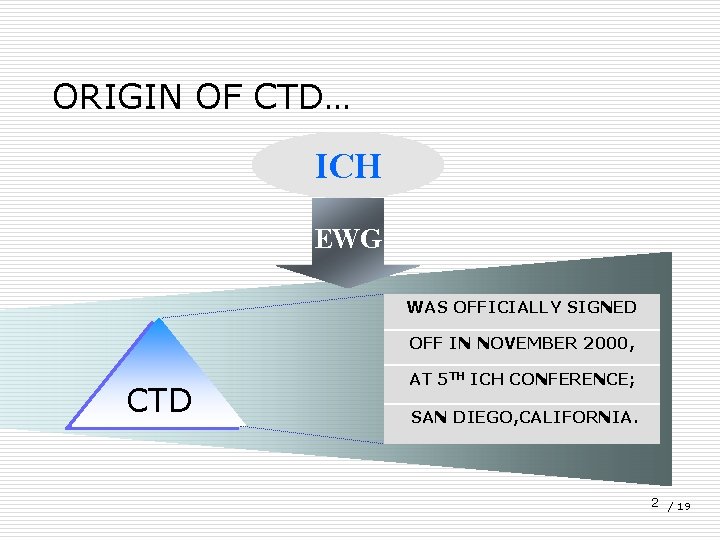
ORIGIN OF CTD… ICH EWG WAS OFFICIALLY SIGNED OFF IN NOVEMBER 2000, CTD AT 5 TH ICH CONFERENCE; SAN DIEGO, CALIFORNIA. 2 / 19

CTD IS A JOINT EFFORT OF 3 REGULATORY AGENCIES: 1. European Medicines Agency (EMEA, Europe), 2. Food and Drug Administration (FDA, USA) and 3. Ministry of Health, Labour and Welfare (MHLW, Japan). CTD is maintained by ICH through EWG. It has been adopted by several other countries including Canada and Switzerland. 3 / 19

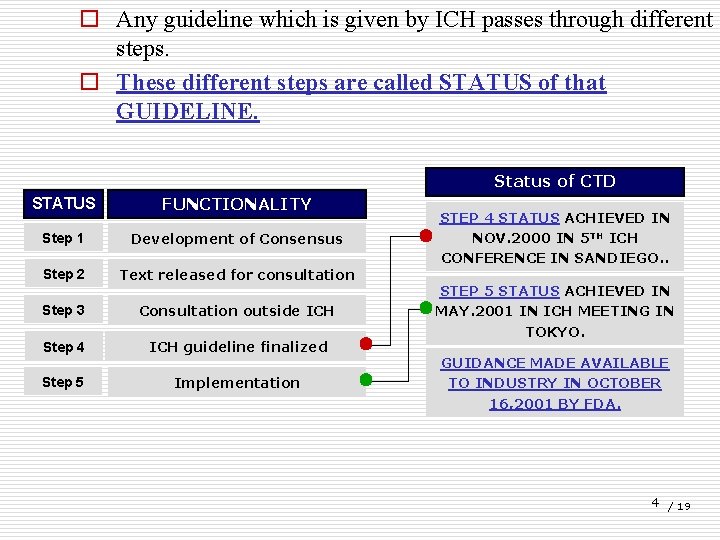
o Any guideline which is given by ICH passes through different steps. o These different steps are called STATUS of that GUIDELINE. Status of CTD STATUS FUNCTIONALITY Step 1 Development of Consensus Step 2 Text released for consultation Step 3 Consultation outside ICH Step 4 ICH guideline finalized Step 5 Implementation STEP 4 STATUS ACHIEVED IN NOV. 2000 IN 5 TH ICH CONFERENCE IN SANDIEGO. . STEP 5 STATUS ACHIEVED IN MAY. 2001 IN ICH MEETING IN TOKYO. GUIDANCE MADE AVAILABLE TO INDUSTRY IN OCTOBER 16, 2001 BY FDA. 4 / 19

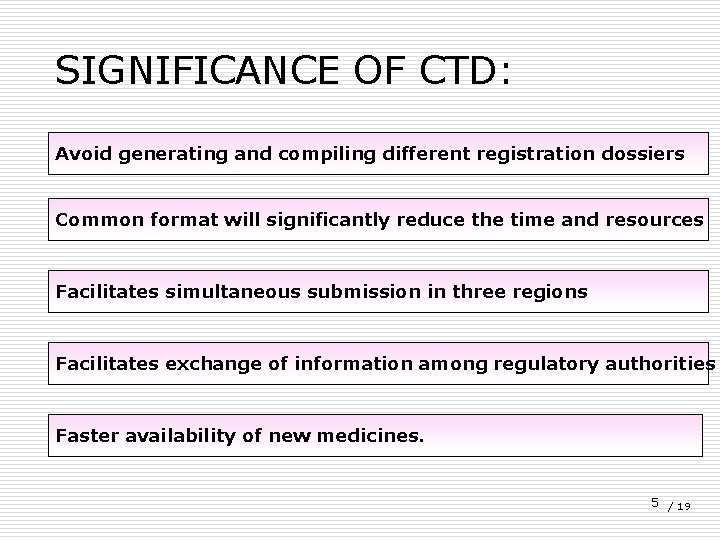
SIGNIFICANCE OF CTD: Avoid generating and compiling different registration dossiers Common format will significantly reduce the time and resources Facilitates simultaneous submission in three regions Facilitates exchange of information among regulatory authorities Faster availability of new medicines. 5 / 19

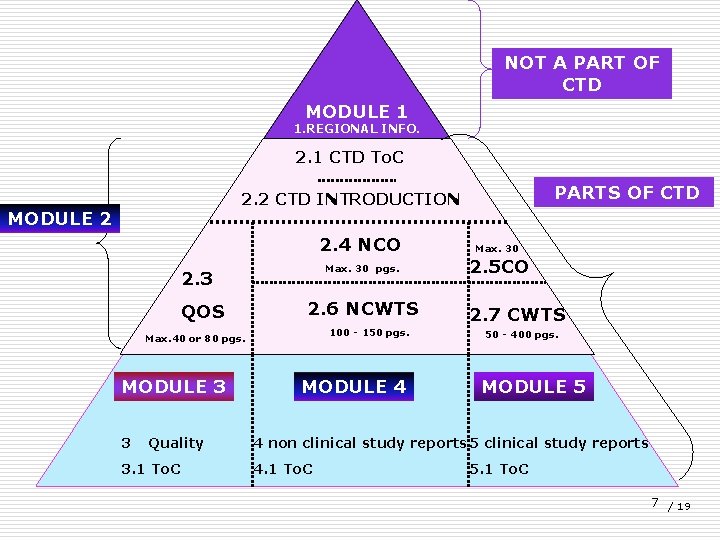
STRUCTURE OF CTD: - Divided into various modules having different contents. 6 / 19

NOT A PART OF CTD MODULE 1 1. REGIONAL INFO. 1. 1 To. C 2. 1 CTD To. C PARTS OF CTD 2. 2 CTD INTRODUCTION MODULE 2 2. 3 QOS 3 Quality 3. 1 To. C Max. 30 pgs. 2. 5 CO 2. 6 NCWTS 100 - 150 pgs. Max. 40 or 80 pgs. MODULE 3 2. 4 NCO MODULE 4 2. 7 CWTS 50 - 400 pgs. MODULE 5 4 non clinical study reports 5 clinical study reports 4. 1 To. C 5. 1 To. C 7 / 19

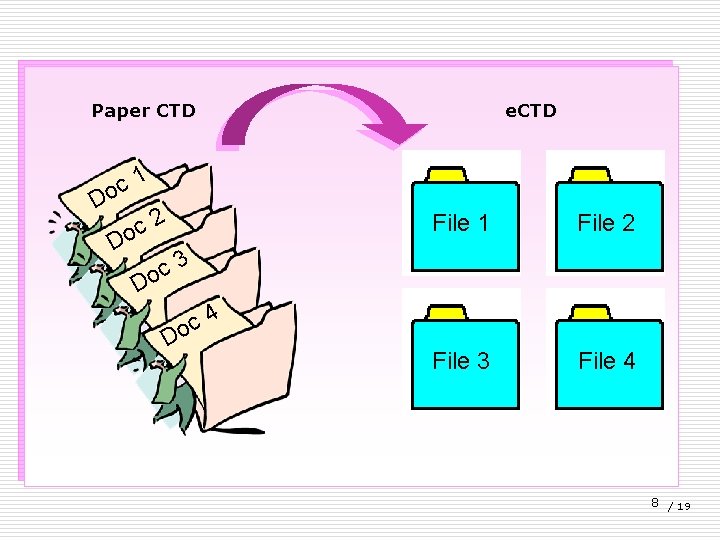
Paper CTD e. CTD 1 c o D 2 c o D File 1 File 2 File 3 File 4 3 c o D D 4 c o 8 / 19

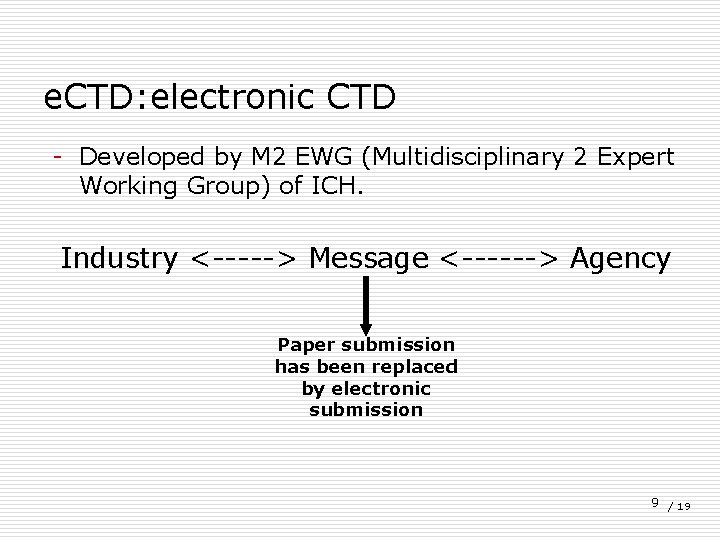
e. CTD: electronic CTD - Developed by M 2 EWG (Multidisciplinary 2 Expert Working Group) of ICH. Industry <-----> Message <------> Agency Paper submission has been replaced by electronic submission 9 / 19

Characteristics of e. CTD: 1. Files Referenced in the XML Backbone(s) (Extensible Markup Language) REASONS: 1. It manages the large data for the entire submission and for each document within the submission. 2. This XML backbone allows the e. CTD submission to be viewed via a web browser and can be loaded on a Web server. 10 / 19

2. The file formats that can be included in the e. CTD are Portable Document Format (PDF) and XML. However other formats can be used for graphs and images. JPEG PNG GIF -may be used for higher resolution. 11 / 19

3. All e. CTD Submissions Include Module 1 Identifies following important information: « « « Company Name Drug Name Submission Type Submission Date Application Number Sequence Number 12 / 19

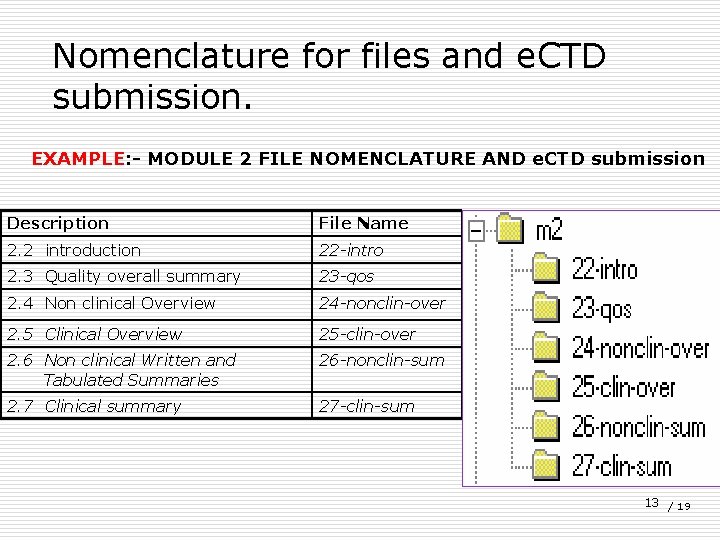
Nomenclature for files and e. CTD submission. EXAMPLE: - MODULE 2 FILE NOMENCLATURE AND e. CTD submission Description File Name 2. 2 introduction 22 -intro 2. 3 Quality overall summary 23 -qos 2. 4 Non clinical Overview 24 -nonclin-over 2. 5 Clinical Overview 25 -clin-over 2. 6 Non clinical Written and Tabulated Summaries 26 -nonclin-sum 2. 7 Clinical summary 27 -clin-sum 13 / 19

Agency Sites with e. CTD submission Information FDA : http: //www. fda. gov/cder/regulatory/ersr/ectd. htm EMEA(EU) : http: //esubmission. eudra. org/ MHLW(JP) : http: //www. mhlw. go. jp/english/index. html 14 / 19

Summary CTD was introduced with the aim to harmonize submission of technical data for registration of human use in different regions. After 5 years of implementation of ctd, we can say that considerable harmonization has been achieved in various regions for submission of technical data. More and more regulatory agencies have started association with this implementation generated actually by ICH before 5 years back. 15 / 19

FOR EXAMPLE : TGA (AUSTRALIAN REGULATORY AGENCY) has started submission of technical data in the form of CTD. http: //www. tga. gov. au/docs/html/eugctd. htm http: //www. tga. gov. au/docs/pdf/euguide/tgamod 1. pdf http: //www. tga. gov. au/docs/pdf/euguide/ich/ctdm 2 quality. pdf http: //www. tga. gov. au/docs/pdf/euguide/ich/ctdm 2 safety. pdf http: //www. tga. gov. au/docs/pdf/euguide/ich/ctdm 2 efficacy. pdf AND TGA IS NOW WORKING ON A PROJECT TO IMPLEMENT e. CTD for marketing authorization, NDA, ANDA AND DIFFERENT DOSSIER SUBMISSION. 16 / 19

Study questions: 1. What is CTD? What are the advantages of it? (uni. exam… 2005) 2. COMMENT ON: ‘CTD is a common guideline for WHO, ICH, MCA, EMEA & USFDA. ’ (uni. exam… 2006) 3. What is CTD? Why it is introduced? Who regulates it? Give diagrammatic representation, advantages and exclusions of it. (uni. exam… 2007) 17 / 19

REFERENCES: üwww. ich. org ühttp: //www. fda. gov/cder/regulatory/ersr/ectd. htm ühttp: //esubmission. eudra. org/ ühttp: //www. mhlw. go. jp/english/index. html ühttp: //www. tga. gov. au/docs/html/eugctd. htm 18 / 19

19