Common Polyatomic Ions Names of Common Polyatomic Ions

![32 Ge 72. 61 Periodic Patterns • Example - Germanium [Ar] 2 4 s 32 Ge 72. 61 Periodic Patterns • Example - Germanium [Ar] 2 4 s](https://slidetodoc.com/presentation_image_h/36ea6278241b920934fa02af26efd659/image-14.jpg)
![Shorthand Configuration Practice Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] Shorthand Configuration Practice Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar]](https://slidetodoc.com/presentation_image_h/36ea6278241b920934fa02af26efd659/image-16.jpg)

![Formation of a Cation sodium atom Na [Ne] 3 s 1 sodium ion Na+ Formation of a Cation sodium atom Na [Ne] 3 s 1 sodium ion Na+](https://slidetodoc.com/presentation_image_h/36ea6278241b920934fa02af26efd659/image-20.jpg)

![Formation of an Anion chlorine atom Cl [Ne]3 s 23 p 5 e- chloride Formation of an Anion chlorine atom Cl [Ne]3 s 23 p 5 e- chloride](https://slidetodoc.com/presentation_image_h/36ea6278241b920934fa02af26efd659/image-22.jpg)








- Slides: 79

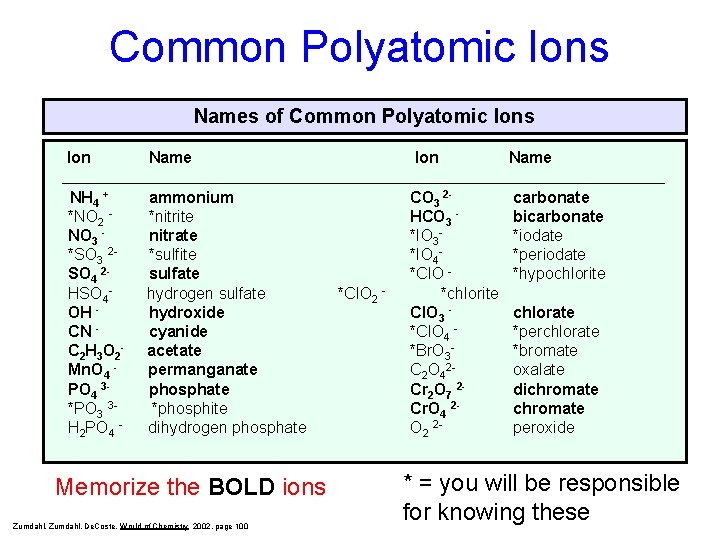
Common Polyatomic Ions Names of Common Polyatomic Ions Ion Name NH 4 + *NO 2 NO 3 *SO 3 2 SO 4 2 HSO 4 OH CN C 2 H 3 O 2 Mn. O 4 PO 4 3*PO 3 3 H 2 PO 4 - ammonium *nitrite nitrate *sulfite sulfate hydrogen sulfate hydroxide cyanide acetate permanganate phosphate *phosphite dihydrogen phosphate CO 3 2 HCO 3 *IO 3*IO 4*Cl. O *chlorite Cl. O 3 *Cl. O 4 *Br. O 3 C 2 O 42 Cr 2 O 7 2 Cr. O 4 2 O 2 2 - carbonate bicarbonate *iodate *periodate *hypochlorite Memorize the BOLD ions Zumdahl, De. Coste, World of Chemistry 2002, page 100 *Cl. O 2 - chlorate *perchlorate *bromate oxalate dichromate peroxide * = you will be responsible for knowing these

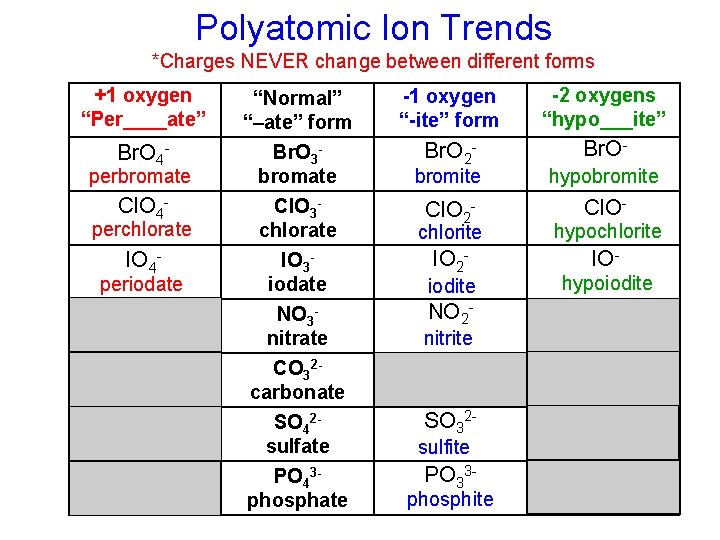
Polyatomic Ion Trends *Charges NEVER change between different forms +1 oxygen “Per____ate” Br. O 4 - perbromate Cl. O 4 - perchlorate IO 4 - periodate “Normal” “–ate” form Br. O 3 bromate Cl. O 3 chlorate -1 oxygen “-ite” form -2 oxygens “hypo___ite” Br. O 2 - Br. O- chlorite hypochlorite IO 3 iodate NO 3 nitrate CO 32 carbonate SO 42 sulfate PO 43 phosphate IO 2 - IO- bromite hypobromite Cl. O 2 - Cl. O- iodite NO 2 - nitrite CO 22 - carbonite SO 32 - sulfite PO 33 - phosphite hypoiodite

Atomic Theory Review

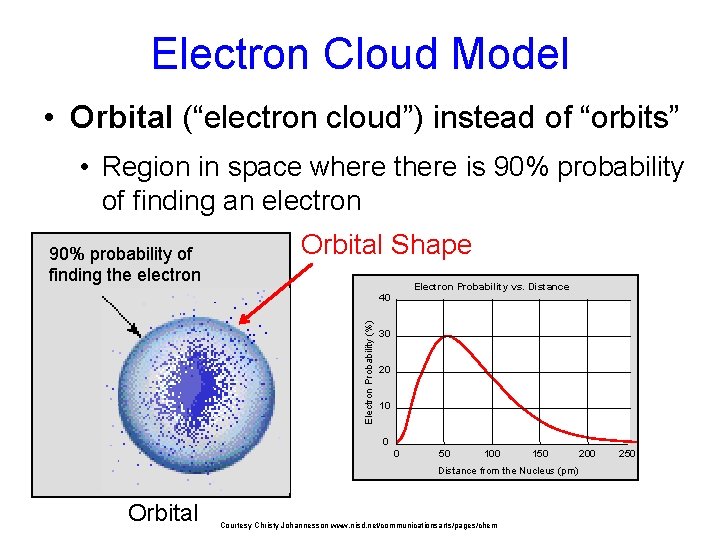
Electron Cloud Model • Orbital (“electron cloud”) instead of “orbits” • Region in space where there is 90% probability of finding an electron 90% probability of finding the electron Orbital Shape Electron Probability vs. Distance Electron Probability (%) 40 30 20 10 0 0 50 100 150 Distance from the Nucleus (pm) Orbital Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 200 250

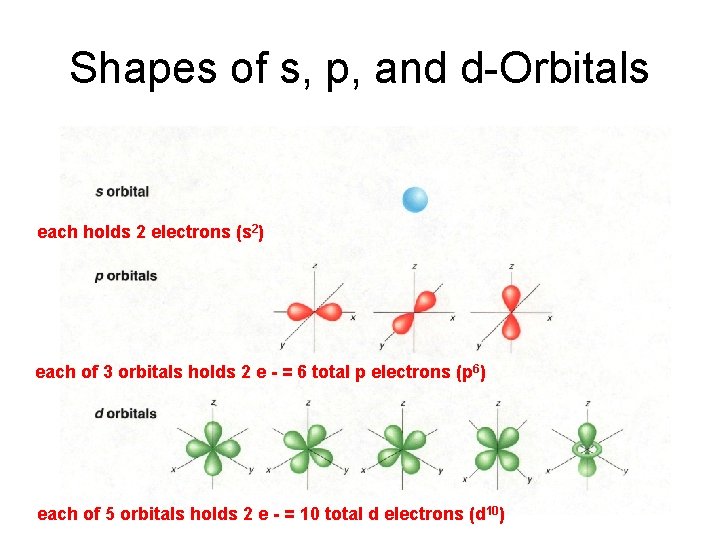
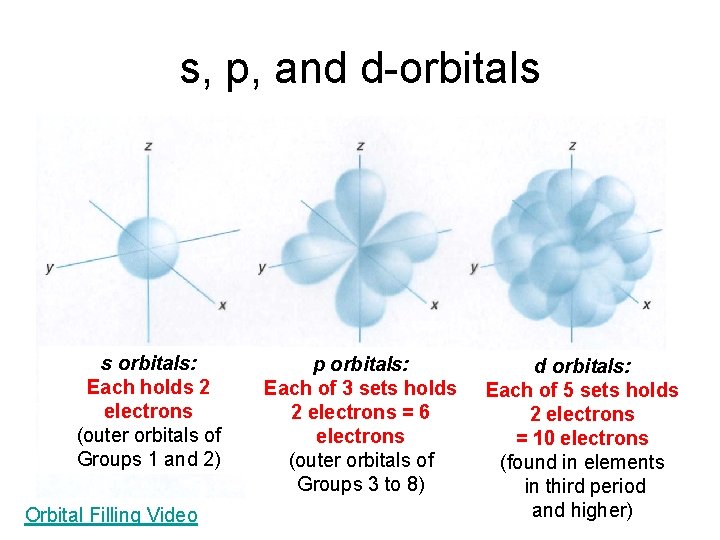
Shapes of s, p, and d-Orbitals each holds 2 electrons (s 2) each of 3 orbitals holds 2 e - = 6 total p electrons (p 6) each of 5 orbitals holds 2 e - = 10 total d electrons (d 10)

s, p, and d-orbitals s orbitals: Each holds 2 electrons (outer orbitals of Groups 1 and 2) Orbital Filling Video p orbitals: Each of 3 sets holds 2 electrons = 6 electrons (outer orbitals of Groups 3 to 8) d orbitals: Each of 5 sets holds 2 electrons = 10 electrons (found in elements in third period and higher)

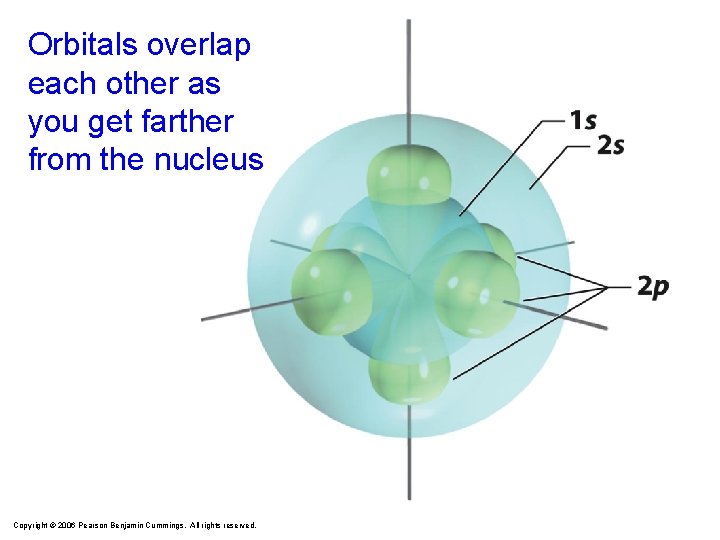
Orbitals overlap each other as you get farther from the nucleus Copyright © 2006 Pearson Benjamin Cummings. All rights reserved.

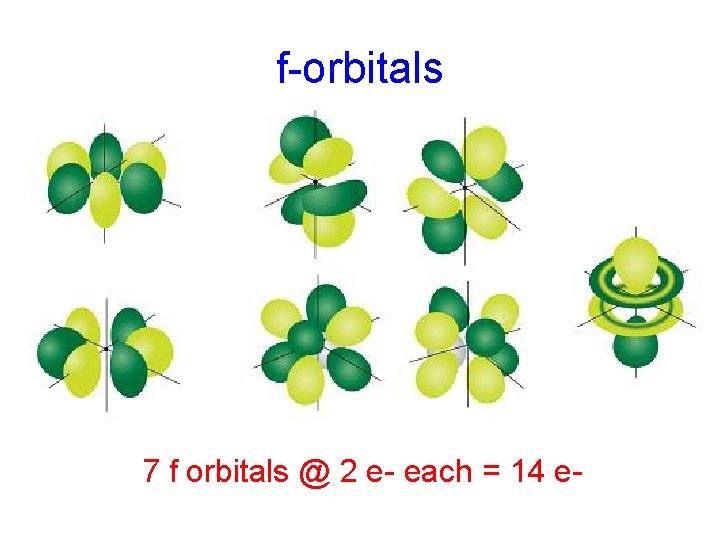
f-orbitals 7 f orbitals @ 2 e- each = 14 e-

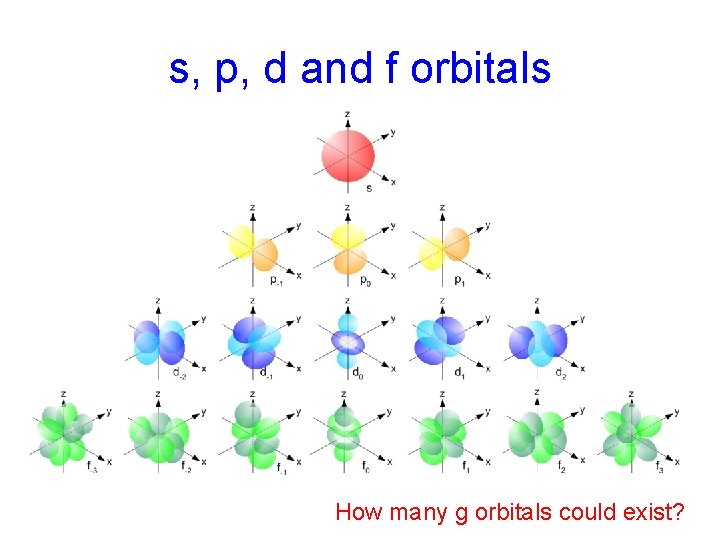
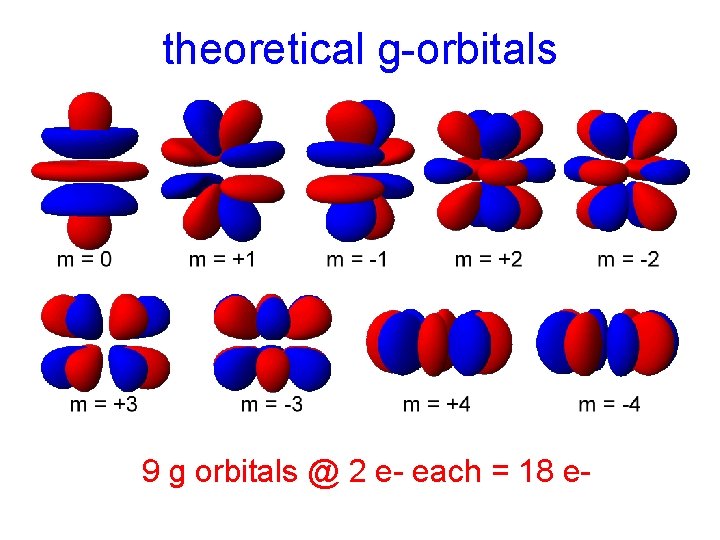
s, p, d and f orbitals How many g orbitals could exist?

theoretical g-orbitals 9 g orbitals @ 2 e- each = 18 e-

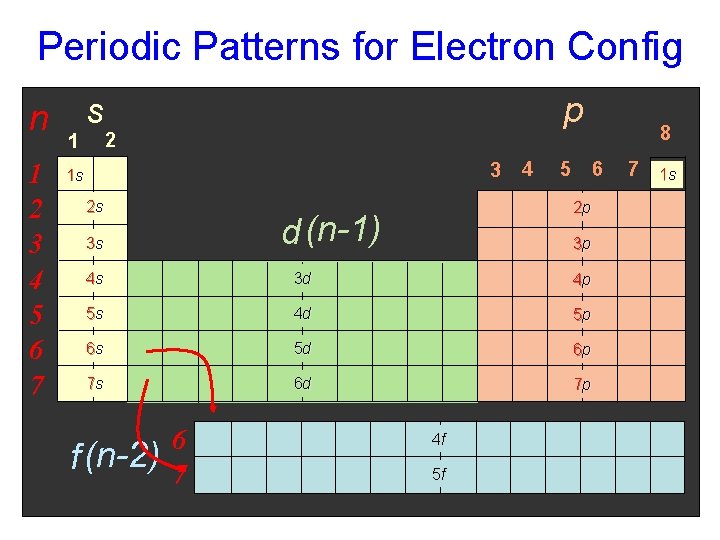
Periodic Patterns for Electron Config n 1 2 3 4 5 6 7 1 s p 2 3 1 s 2 s 6 5 2 p d (n-1) 3 s 4 3 p 4 s 3 d 4 p 5 s 4 d 5 p 6 s 5 d 6 p 7 s 6 d 7 p f (n-2) 6 7 4 f 5 f 8 7 1 s

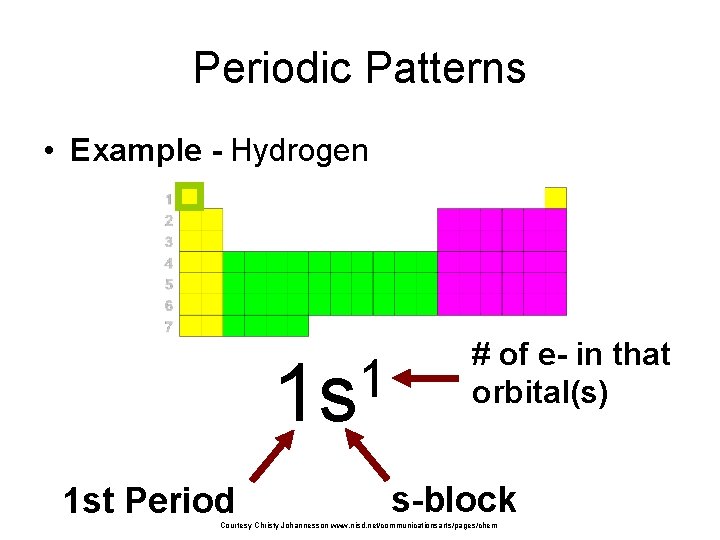
Periodic Patterns • Example - Hydrogen 1 1 s 1 st Period # of e- in that orbital(s) s-block Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

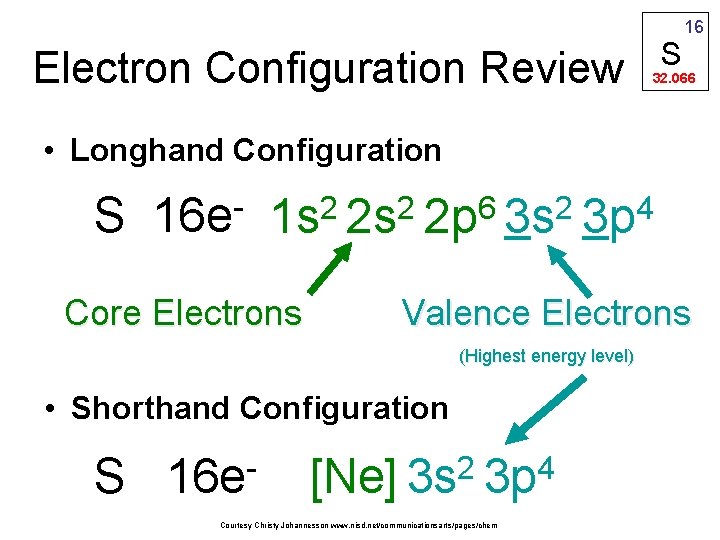
Electron Configuration Review S 16 32. 066 • Longhand Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons 4 3 p Valence Electrons (Highest energy level) • Shorthand Configuration S 16 e 2 4 [Ne] 3 s 3 p Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
![32 Ge 72 61 Periodic Patterns Example Germanium Ar 2 4 s 32 Ge 72. 61 Periodic Patterns • Example - Germanium [Ar] 2 4 s](https://slidetodoc.com/presentation_image_h/36ea6278241b920934fa02af26efd659/image-14.jpg)
32 Ge 72. 61 Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 2 4 p

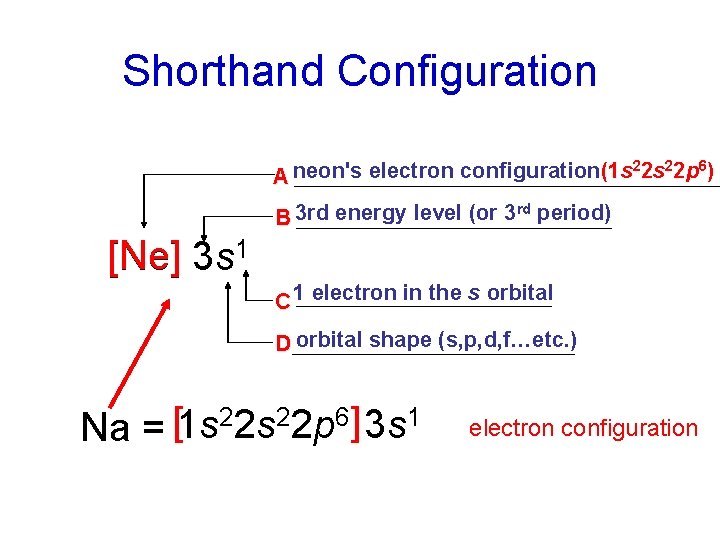
Shorthand Configuration 22 s 22 p 6) neon's electron configuration(1 s A rd B 3 rd energy level (or 3 period) [Ne] 3 s 1 C 1 electron in the s orbital D orbital shape (s, p, d, f…etc. ) 22 s 22 p 6] 3 s 1 [ 1 s Na = electron configuration
![Shorthand Configuration Practice Element symbol Electron configuration Ca Ar 4 s 2 V Ar Shorthand Configuration Practice Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar]](https://slidetodoc.com/presentation_image_h/36ea6278241b920934fa02af26efd659/image-16.jpg)
Shorthand Configuration Practice Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4 s 2 3 d 3 F [He] 2 s 2 2 p 5 Ag [Kr] 5 s 2 4 d 9 I [Kr] 5 s 2 4 d 10 5 p 5 Xe [Kr] 5 s 2 4 d 10 5 p 6 Fe Sg 22 p 64 s [He] 2 s[Ar] 3 s 223 d 3 p 664 s 23 d 6 [Rn] 7 s 2 5 f 14 6 d 4

Periodic Patterns Review • Period # (1 -7) • energy level • (subtract for d & f) • Group # (1 -8…excluding d block) • total # of valence e- • Column within Sublevel block • # of e- in sublevel/orbital Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

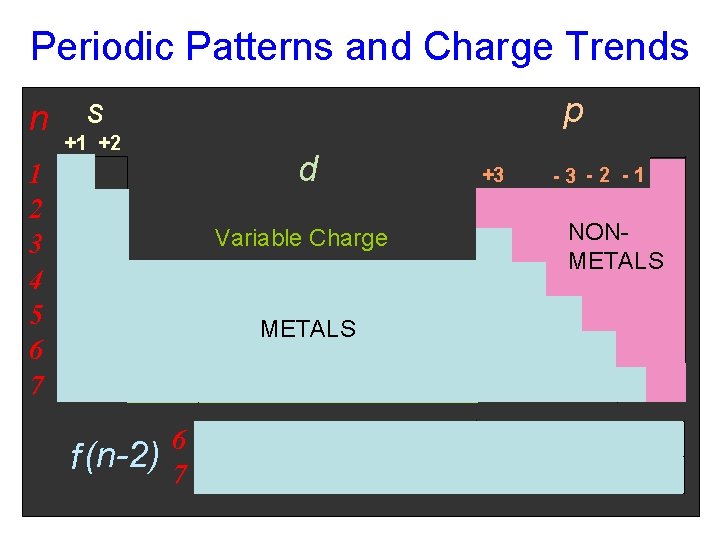
Periodic Patterns and Charge Trends n p s +1 +2 d 1 2 3 4 5 6 7 Variable Charge METALS f (n-2) 6 7 +3 -3 -2 -1 1 s NONMETALS

Electron Configurations for Cations • Metals lose e- to attain a noble gas configuration • Metals form positive ions – Cations are “paws”itive • Metal ions will lose e- from configuration Na: 1 s 22 p 63 s 1 Na+: 1 s 22 p 6 noble gas configuration
![Formation of a Cation sodium atom Na Ne 3 s 1 sodium ion Na Formation of a Cation sodium atom Na [Ne] 3 s 1 sodium ion Na+](https://slidetodoc.com/presentation_image_h/36ea6278241b920934fa02af26efd659/image-20.jpg)
Formation of a Cation sodium atom Na [Ne] 3 s 1 sodium ion Na+ [Ne] e- e- e- 11 p+ ee- loss of one valence electron e- e- 11 p+ e- e- ee-

Electron Configurations for Anions • Non-metals gain electrons to attain a noble gas configuration • They form negative ions • Take a look at the e- configuration S 1 s 22 p 63 s 23 p 4 (6 valence electrons) + 2 e. S 2 - 1 s 22 p 63 s 23 p 6 (noble gas configuration)
![Formation of an Anion chlorine atom Cl Ne3 s 23 p 5 e chloride Formation of an Anion chlorine atom Cl [Ne]3 s 23 p 5 e- chloride](https://slidetodoc.com/presentation_image_h/36ea6278241b920934fa02af26efd659/image-22.jpg)
Formation of an Anion chlorine atom Cl [Ne]3 s 23 p 5 e- chloride ion Cl – [Ne]3 s 23 p 6 or [Ar] egain of one valence electron ee- e- e- ee- e- 17 p+ e- e- ee- e- e- eee- e- e-

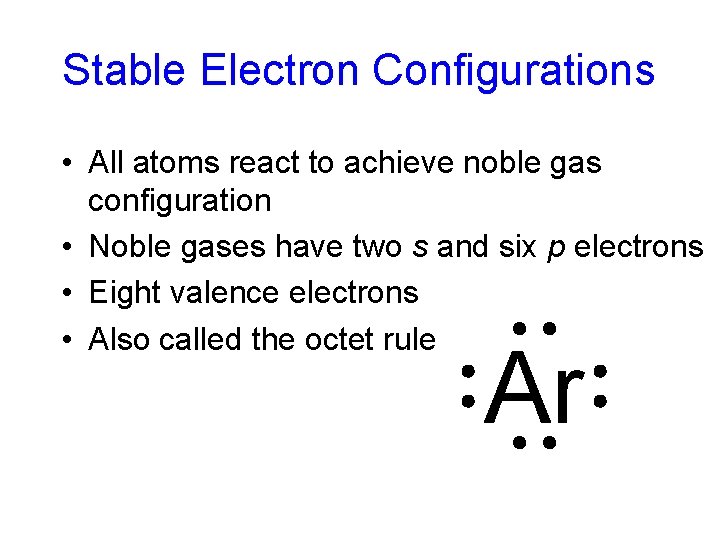
Stable Electron Configurations • All atoms react to achieve noble gas configuration • Noble gases have two s and six p electrons • Eight valence electrons • Also called the octet rule Ar

Table salt Bonding Review and Writing Ionic Formulas

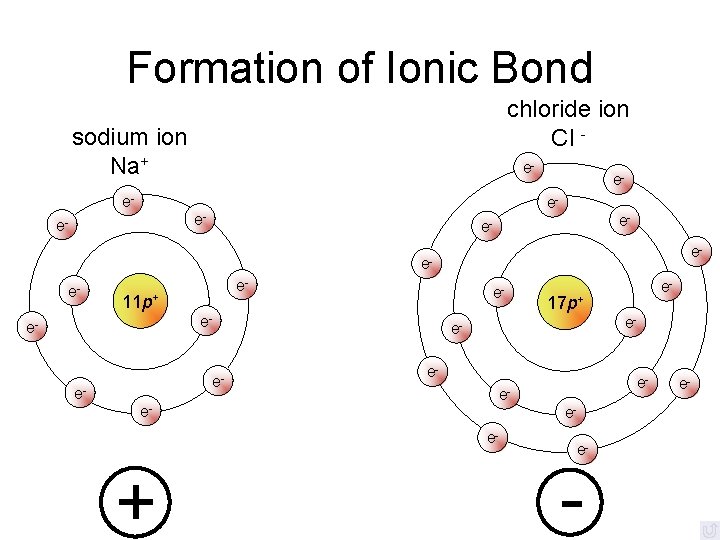
Formation of Ionic Bond chloride ion Cl - sodium ion Na+ e- e- e- ee- e- 11 p+ e- e- ee- 17 p+ e- e- eee- + e- e-

Ionic Bonding • Anions and cations are held together by opposite charges • All ionic compounds are called salts • Simplest ratio is called the formula unit • The bond is formed through the transfer of electrons • Electrons are transferred to fill available orbitals, or achieve a noble gas configuration

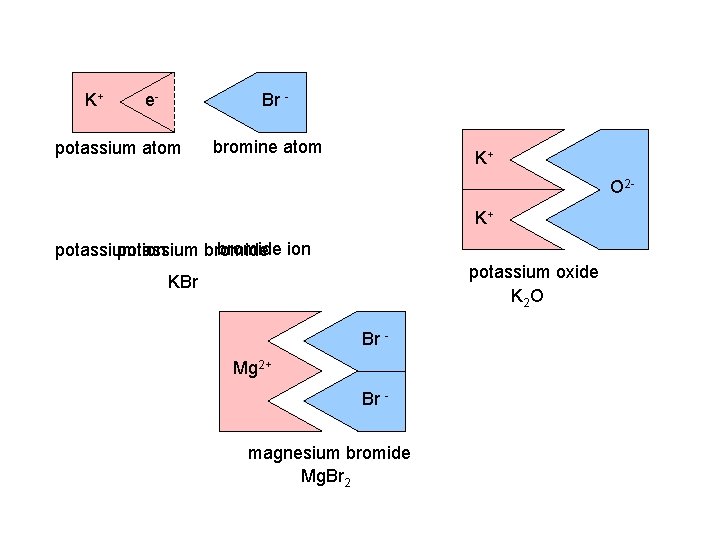
K+ e- e- potassium atom Br. Br- bromine atom K+ O 2 K+ bromide ion potassium ion bromide potassium oxide K 2 O KBr Br Mg 2+ Br magnesium bromide Mg. Br 2

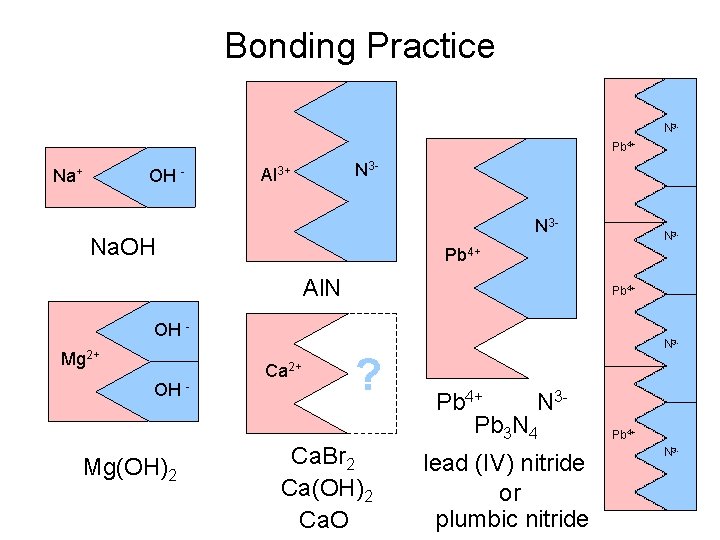
Bonding Practice N 3 Pb 4+ Na+ OH - N 3 - Al 3+ N 3 - Na. OH N 3 - Pb 4+ Al. N Pb 4+ OH Mg 2+ OH - Mg(OH)2 Ca 2+ ? Ca. Br 2 Ca(OH)2 Ca. O N 3 - Pb 4+ N 3 Pb 3 N 4 lead (IV) nitride or plumbic nitride Pb 4+ N 3 -

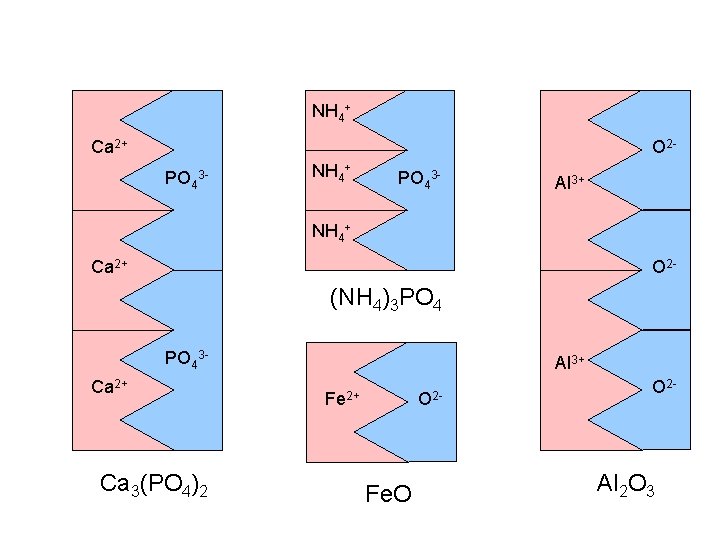
NH 4+ Ca 2+ O 2 PO 4 3 - NH 4+ PO 43 - Al 3+ NH 4+ Ca 2+ O 2 - (NH 4)3 PO 43 Ca 2+ Ca 3(PO 4)2 Al 3+ Fe 2+ O 2 - Fe. O O 2 - Al 2 O 3

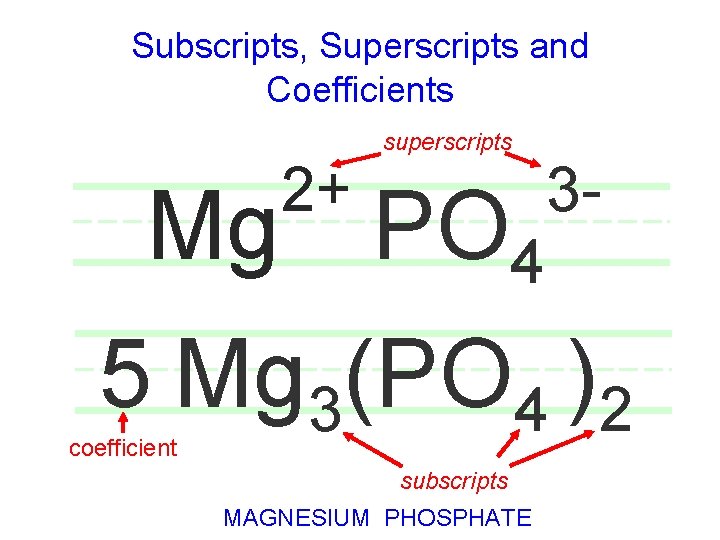
Subscripts, Superscripts and Coefficients 2+ superscripts Mg PO 4 3 - 5 Mg 3(PO 4 )2 coefficient subscripts MAGNESIUM PHOSPHATE

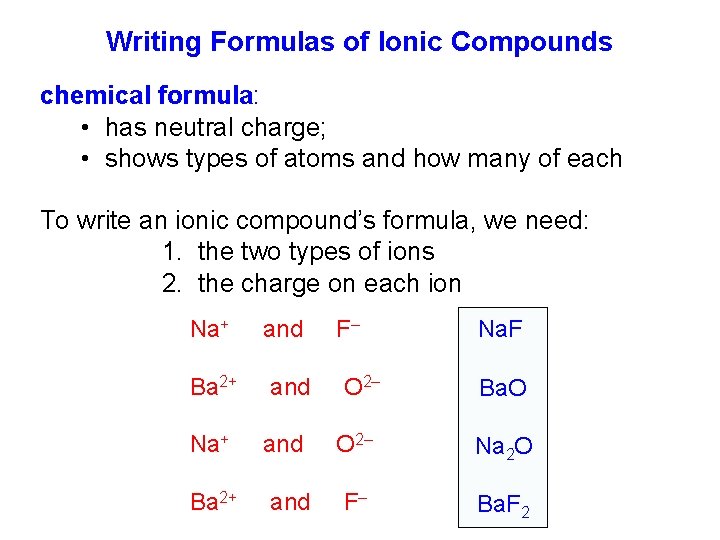
Writing Formulas of Ionic Compounds chemical formula: • has neutral charge; • shows types of atoms and how many of each To write an ionic compound’s formula, we need: 1. the two types of ions 2. the charge on each ion Na+ Ba 2+ and and F– O 2– Na. F Ba. O O 2– Na 2 O F– Ba. F 2

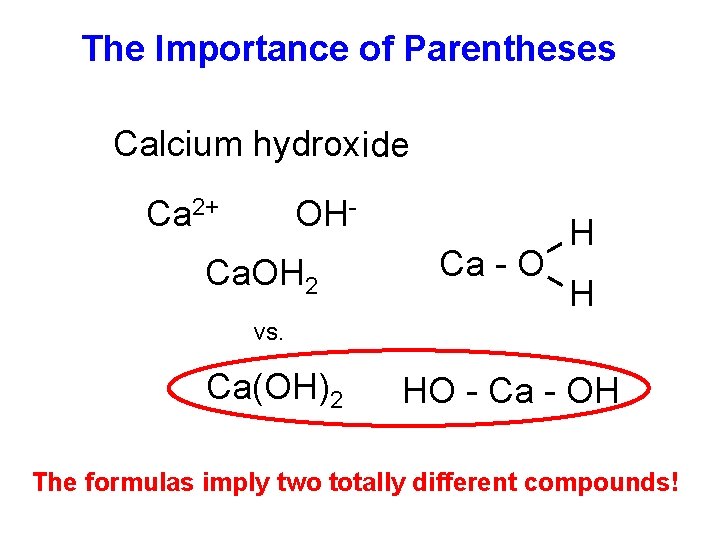
The Importance of Parentheses Calcium hydroxide Ca 2+ OH- Ca. OH 2 Ca - O H H vs. Ca(OH)2 HO - Ca - OH The formulas imply two totally different compounds!

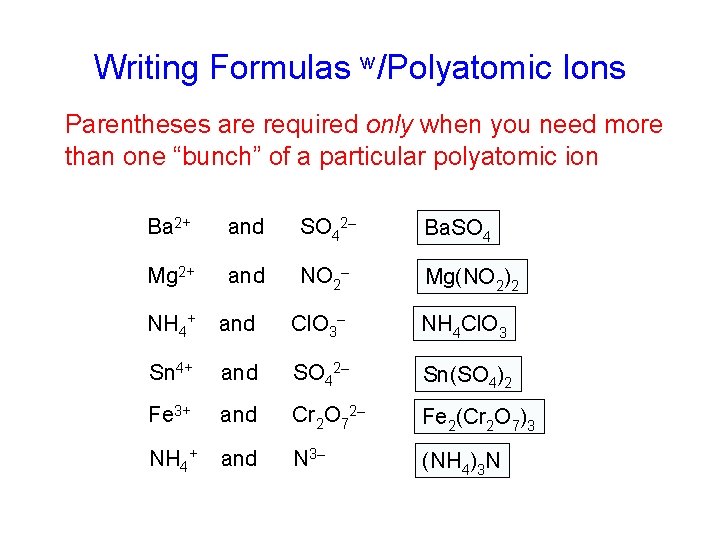
Writing Formulas w/Polyatomic Ions Parentheses are required only when you need more than one “bunch” of a particular polyatomic ion Ba 2+ and SO 42– Ba. SO 4 Mg 2+ and NO 2– Mg(NO 2)2 NH 4+ and Cl. O 3– NH 4 Cl. O 3 Sn 4+ and SO 42– Sn(SO 4)2 Fe 3+ and Cr 2 O 72– Fe 2(Cr 2 O 7)3 NH 4+ and N 3– (NH 4)3 N

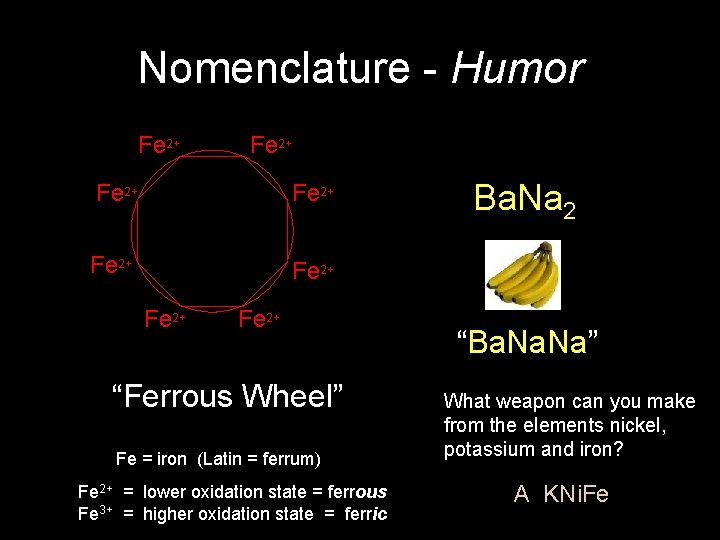
Nomenclature - Humor Fe 2+ Fe 2+ “Ferrous Wheel” Fe = iron (Latin = ferrum) Fe 2+ = lower oxidation state = ferrous Fe 3+ = higher oxidation state = ferric Ba. Na 2 “Ba. Na” What weapon can you make from the elements nickel, potassium and iron? A KNi. Fe

Teacher: What is the formula for water? Student: H, I, J, K, L, M, N, O Teacher: That’s not what I taught you. Student: But you said the formula for water was…H to O. "H-O-H"? ! WHAT'S THAT SPELL? ! mis WATER? Website: Dihydrogen monoxide Information Campaign “Little Johnny took a drink, Now he shall drink no more. For what he thought was H 2 O, Was H 2 SO 4. ” Under aged Pb walks into a bar and the bartender turns to the gold Bouncer and says, “Au, get the lead out!”

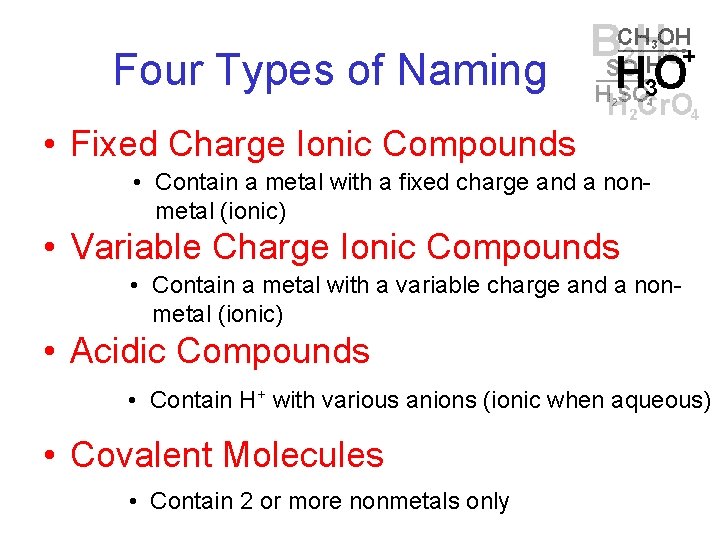
Four Types of Naming • Fixed Charge Ionic Compounds • Contain a metal with a fixed charge and a nonmetal (ionic) • Variable Charge Ionic Compounds • Contain a metal with a variable charge and a nonmetal (ionic) • Acidic Compounds • Contain H+ with various anions (ionic when aqueous) • Covalent Molecules • Contain 2 or more nonmetals only

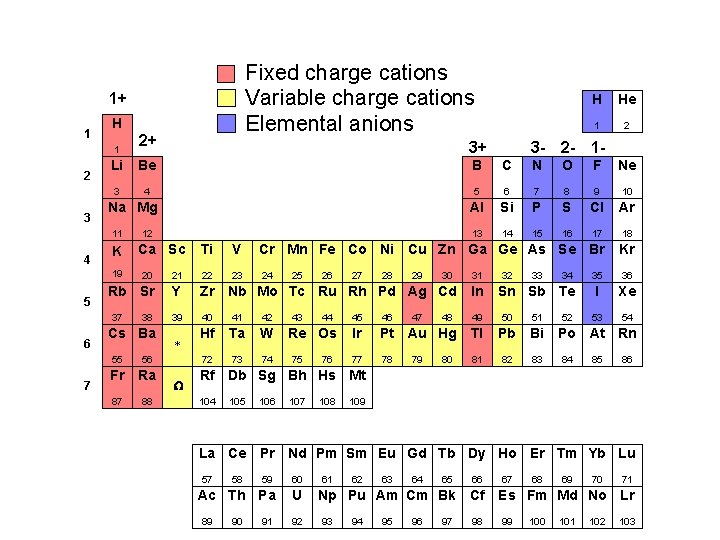
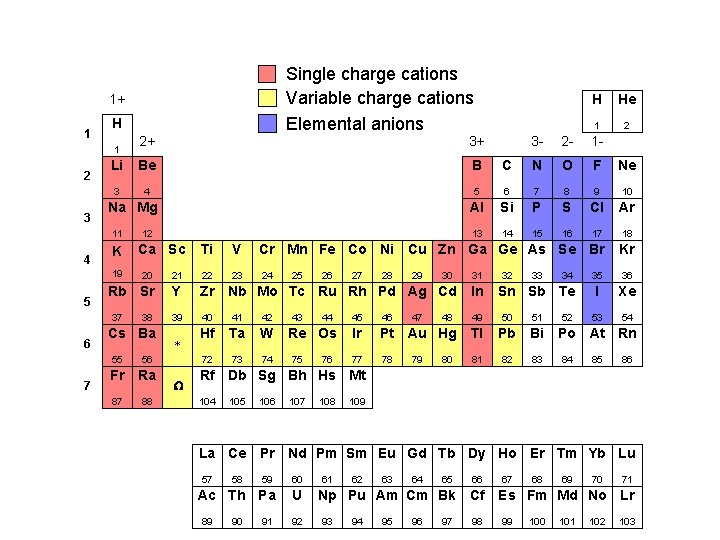
Fixed charge cations Variable charge cations Elemental anions 1+ 1 H 1 2 3 7 2 3 - 2 - 1 - B C N O F Ne 3 4 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Na Mg K 12 Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 20 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 37 6 1 Be 19 5 3+ He Li 11 4 2+ H 38 Cs Ba 55 56 Fr Ra 87 88 * W 25 43 26 44 Re Os 75 76 27 28 29 47 30 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 34 Sn Sb Te 45 79 48 31 52 Po At Rn 84 85 86 Rf Db Sg Bh Hs Mt 104 105 106 107 108 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 59 60 Ac Th Pa U 89 58 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103

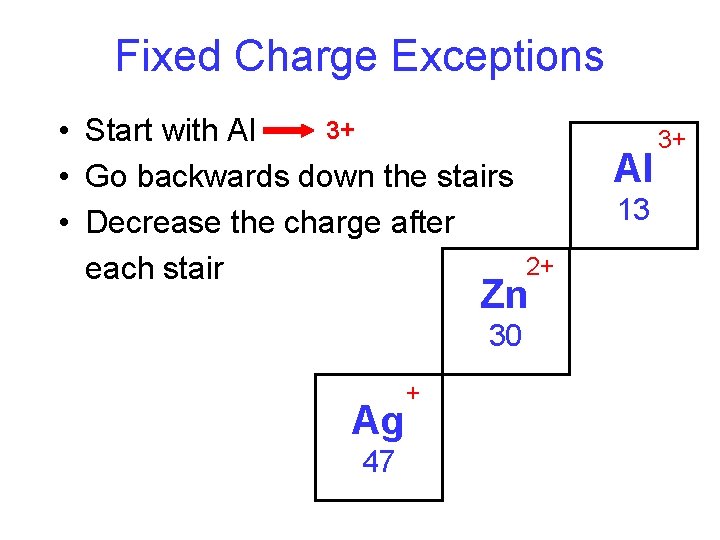
Fixed Charge Exceptions 3+ • Start with Al • Go backwards down the stairs • Decrease the charge after each stair Al 13 2+ Zn 30 Ag 47 + 3+

Fixed Charge Compound Nomenclature Metals (fixed charge) + Nonmetals

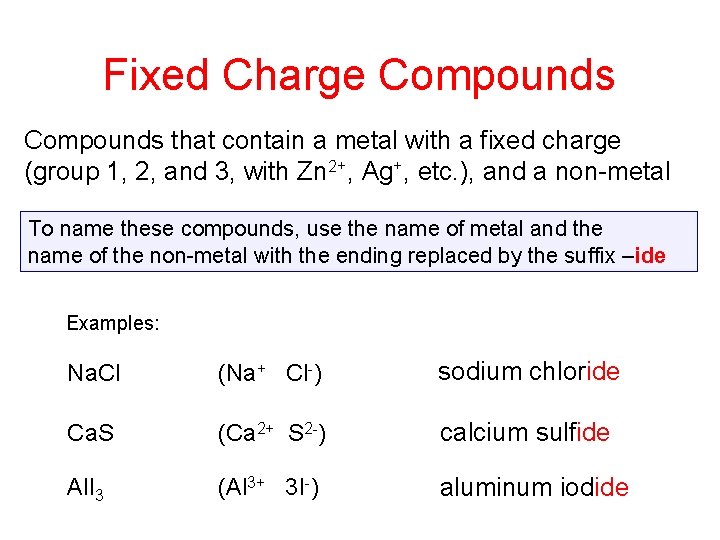
Fixed Charge Compounds that contain a metal with a fixed charge (group 1, 2, and 3, with Zn 2+, Ag+, etc. ), and a non-metal To name these compounds, use the name of metal and the name of the non-metal with the ending replaced by the suffix –ide Examples: Na. Cl (Na+ Cl-) sodium chloride Ca. S (Ca 2+ S 2 -) calcium sulfide Al. I 3 (Al 3+ 3 I-) aluminum iodide

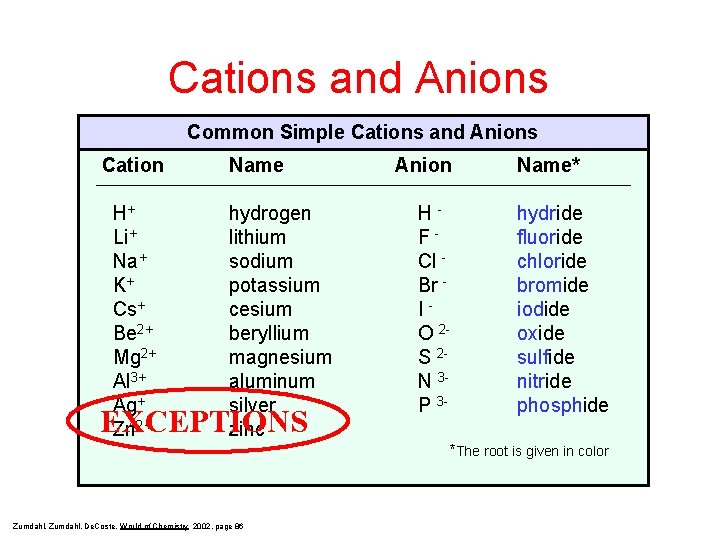
Cations and Anions Common Simple Cations and Anions Cation H+ Li+ Na+ K+ Cs+ Be 2+ Mg 2+ Al 3+ Ag+ Zn 2+ Name hydrogen lithium sodium potassium cesium beryllium magnesium aluminum silver zinc EXCEPTIONS Anion HFCl Br IO 2 S 2 N 3 P 3 - Name* hydride fluoride chloride bromide iodide oxide sulfide nitride phosphide *The root is given in color Zumdahl, De. Coste, World of Chemistry 2002, page 86

“Perhaps one of you gentlemen would mind telling me just what is outside the window that you find so attractive. . ? ” Image courtesy Nearing. Zero. net

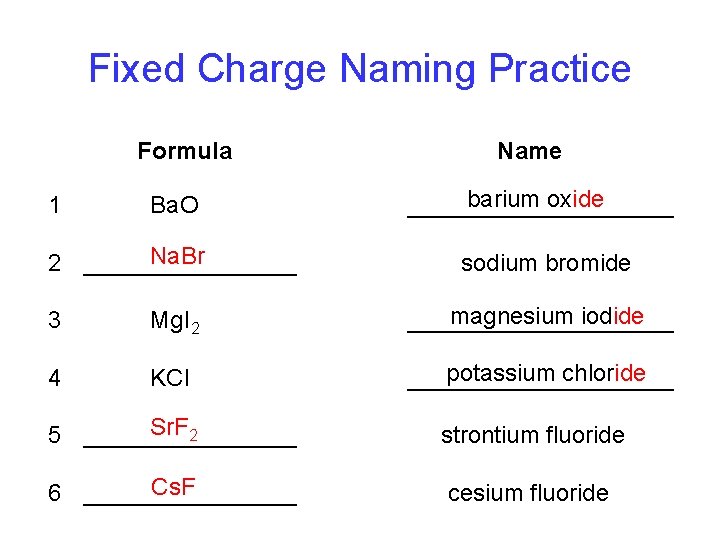
Fixed Charge Naming Practice Formula Name Ba. O barium oxide __________ Na. Br 2 ________ sodium bromide 1 3 Mg. I 2 magnesium iodide __________ 4 KCl potassium chloride __________ Sr. F 2 5 ________ strontium fluoride Cs. F 6 ________ cesium fluoride

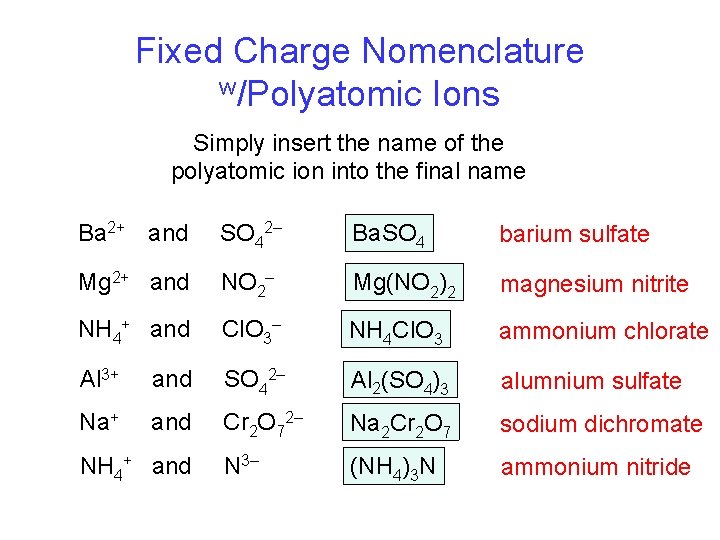
Fixed Charge Nomenclature w/Polyatomic Ions Simply insert the name of the polyatomic ion into the final name Ba 2+ and SO 42– Ba. SO 4 barium sulfate Mg 2+ and NO 2– Mg(NO 2)2 magnesium nitrite NH 4+ and Cl. O 3– NH 4 Cl. O 3 ammonium chlorate Al 3+ and SO 42– Al 2(SO 4)3 alumnium sulfate Na+ and Cr 2 O 72– Na 2 Cr 2 O 7 sodium dichromate N 3– (NH 4)3 N ammonium nitride NH 4+ and

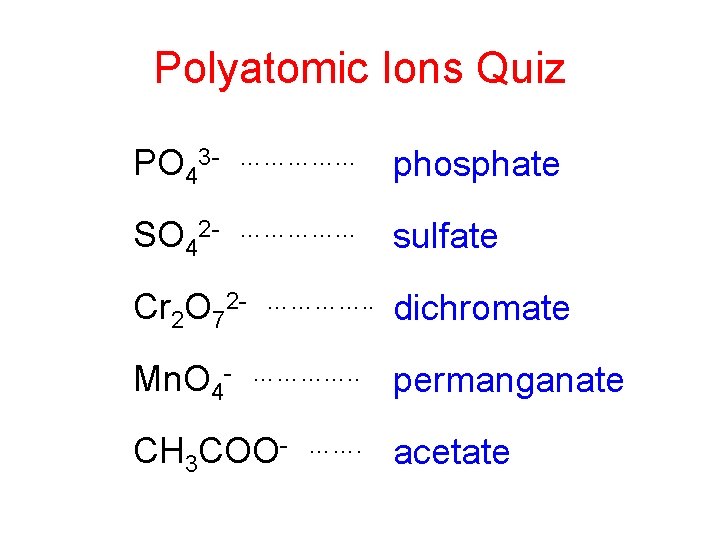
Polyatomic Ions Quiz PO 43 - …………… phosphate SO 42 - …………… sulfate Cr 2 O 72 Mn. O 4 - …………. . CH 3 COO- ……. dichromate permanganate acetate

Variable Charge Compound Nomenclature Metals (variable charge) + Nonmetals

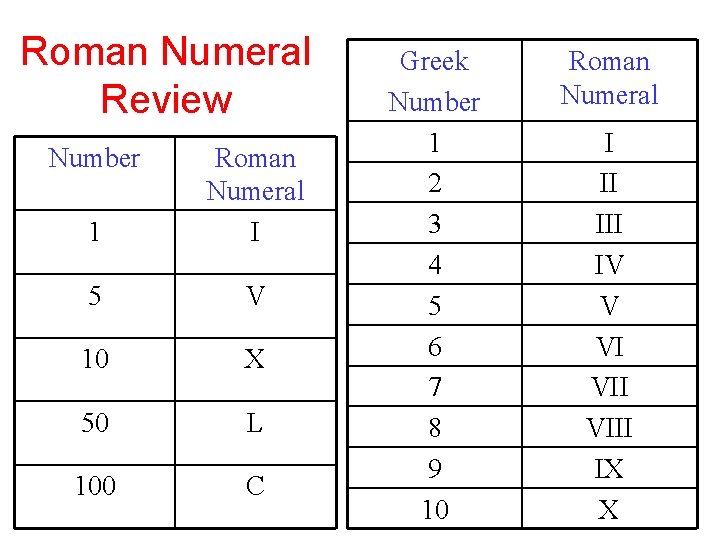
Roman Numeral Review Number 1 Roman Numeral I 5 V 10 X 50 L 100 C Greek Number 1 2 3 4 5 6 7 8 9 10 Roman Numeral I II IV V VI VIII IX X

Single charge cations Variable charge cations Elemental anions 1+ 1 H 2+ 3+ Li Be B 3 4 1 2 3 Na Mg 11 4 K 19 5 7 Ca Sc C N O F Ne 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 55 56 Fr Ra 87 88 * W 2 1 - 22 Cs Ba 1 2 - 21 38 He 3 - 20 37 6 12 H 25 43 26 44 Re Os 75 76 27 28 29 47 30 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 34 Sn Sb Te 45 79 48 31 52 Po At Rn 84 85 86 Rf Db Sg Bh Hs Mt 104 105 106 107 108 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 59 60 Ac Th Pa U 89 58 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103

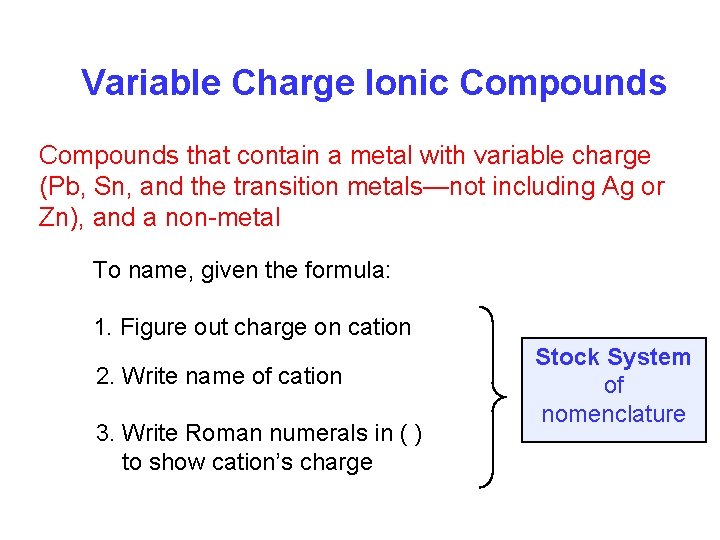
Variable Charge Ionic Compounds that contain a metal with variable charge (Pb, Sn, and the transition metals—not including Ag or Zn), and a non-metal To name, given the formula: 1. Figure out charge on cation 2. Write name of cation 3. Write Roman numerals in ( ) to show cation’s charge Stock System of nomenclature

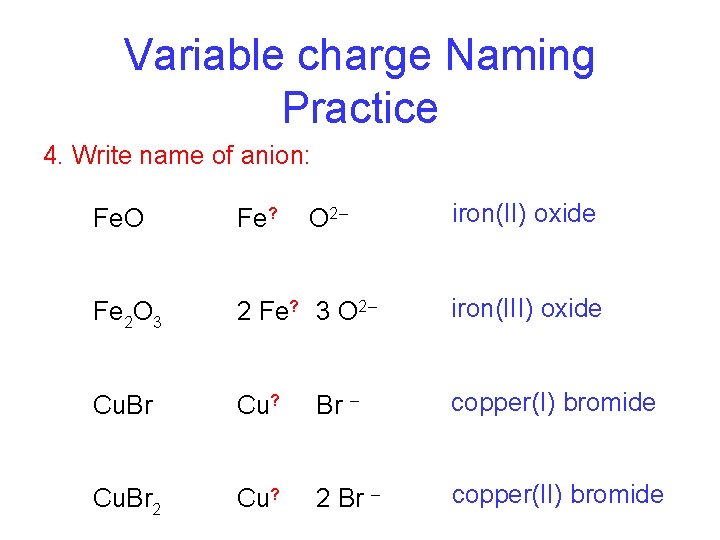
Variable charge Naming Practice 4. Write name of anion: O 2– iron(II) oxide Fe. O Fe? Fe 2 O 3 2 Fe? 3 O 2– iron(III) oxide Cu. Br Cu? Br – copper(I) bromide Cu. Br 2 Cu? 2 Br – copper(II) bromide

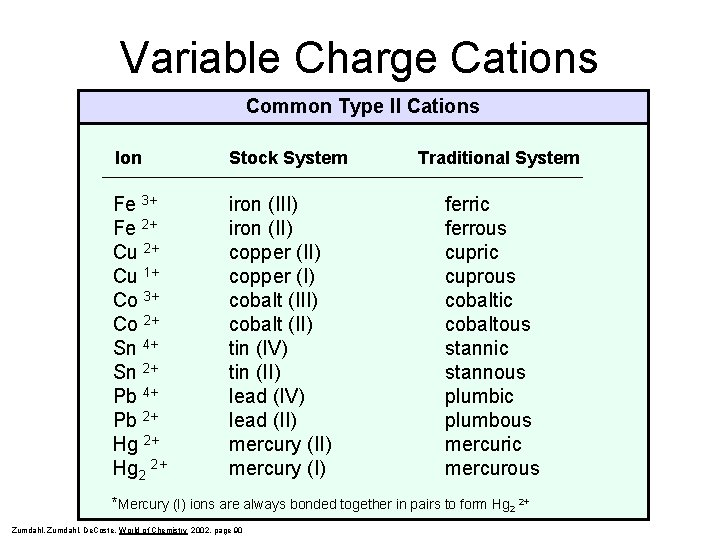
Variable Charge Cations Common Type II Cations Ion Stock System Fe 3+ Fe 2+ Cu 1+ Co 3+ Co 2+ Sn 4+ Sn 2+ Pb 4+ Pb 2+ Hg 2 2+ iron (III) iron (II) copper (I) cobalt (II) tin (IV) tin (II) lead (IV) lead (II) mercury (I) Traditional System ferric ferrous cupric cuprous cobaltic cobaltous stannic stannous plumbic plumbous mercuric mercurous *Mercury (I) ions are always bonded together in pairs to form Hg 2 2+ Zumdahl, De. Coste, World of Chemistry 2002, page 90

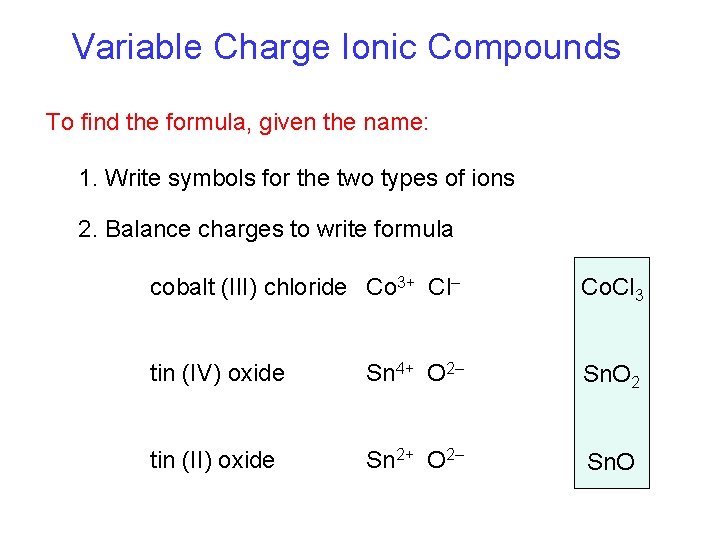
Variable Charge Ionic Compounds To find the formula, given the name: 1. Write symbols for the two types of ions 2. Balance charges to write formula cobalt (III) chloride Co 3+ Cl– Co. Cl 3 tin (IV) oxide Sn 4+ O 2– Sn. O 2 tin (II) oxide Sn 2+ O 2– Sn. O

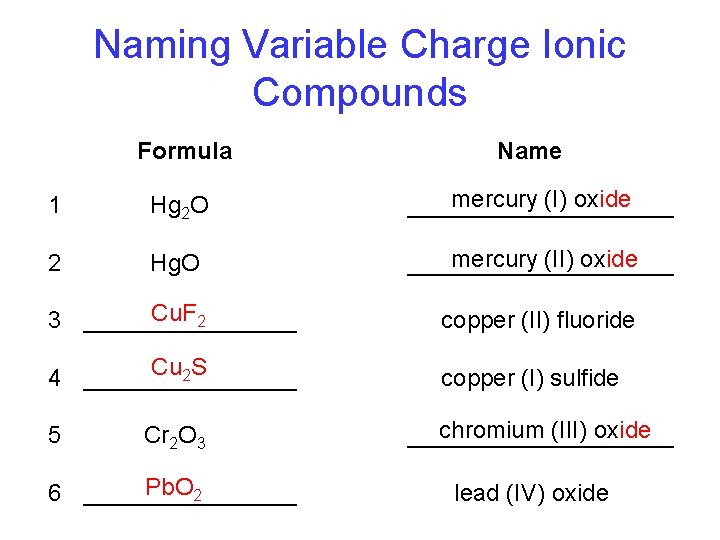
Naming Variable Charge Ionic Compounds Formula Name 1 Hg 2 O mercury (I) oxide __________ 2 Hg. O mercury (II) oxide __________ Cu. F 2 3 ________ copper (II) fluoride Cu 2 S 4 ________ copper (I) sulfide 5 Cr 2 O 3 Pb. O 2 6 ________ chromium (III) oxide __________ lead (IV) oxide

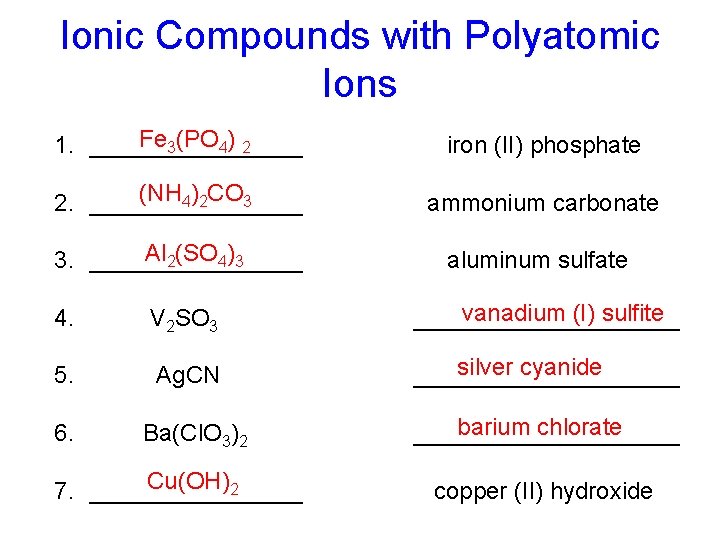
Ionic Compounds with Polyatomic Ions Fe 3(PO 4) 2 1. ________ iron (II) phosphate (NH 4)2 CO 3 2. ________ ammonium carbonate Al 2(SO 4)3 3. ________ aluminum sulfate 4. V 2 SO 3 vanadium (I) sulfite __________ 5. Ag. CN silver cyanide __________ 6. Ba(Cl. O 3)2 barium chlorate __________ Cu(OH)2 7. ________ copper (II) hydroxide

Acidic Compounds Oxysalts + H 2 O Oxyacids

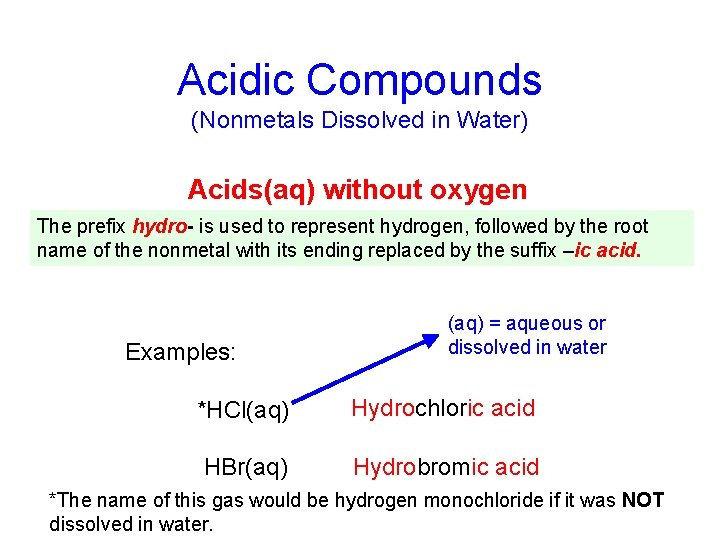
Acidic Compounds (Nonmetals Dissolved in Water) Acids(aq) without oxygen The prefix hydro- is used to represent hydrogen, followed by the root name of the nonmetal with its ending replaced by the suffix –ic acid. Examples: (aq) = aqueous or dissolved in water *HCl(aq) Hydrochloric acid HBr(aq) Hydrobromic acid *The name of this gas would be hydrogen monochloride if it was NOT dissolved in water.

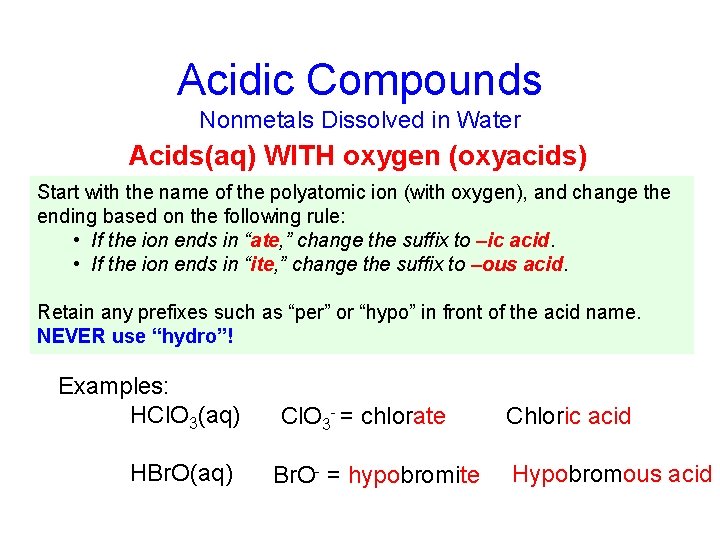
Acidic Compounds Nonmetals Dissolved in Water Acids(aq) WITH oxygen (oxyacids) Start with the name of the polyatomic ion (with oxygen), and change the ending based on the following rule: • If the ion ends in “ate, ” change the suffix to –ic acid. • If the ion ends in “ite, ” change the suffix to –ous acid. Retain any prefixes such as “per” or “hypo” in front of the acid name. NEVER use “hydro”! Examples: HCl. O 3(aq) HBr. O(aq) Cl. O 3 - = chlorate Br. O- = hypobromite Chloric acid Hypobromous acid

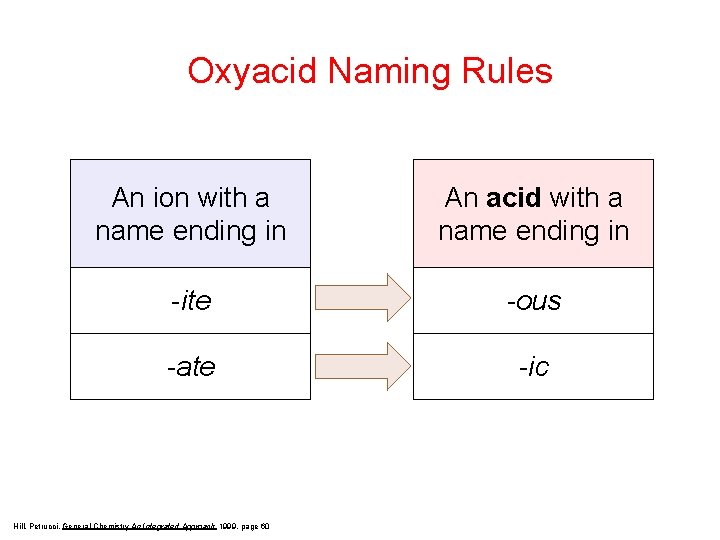
Oxyacid Naming Rules An ion with a name ending in An acid with a name ending in -ite -ous -ate -ic Hill, Petrucci, General Chemistry An Integrated Approach 1999, page 60

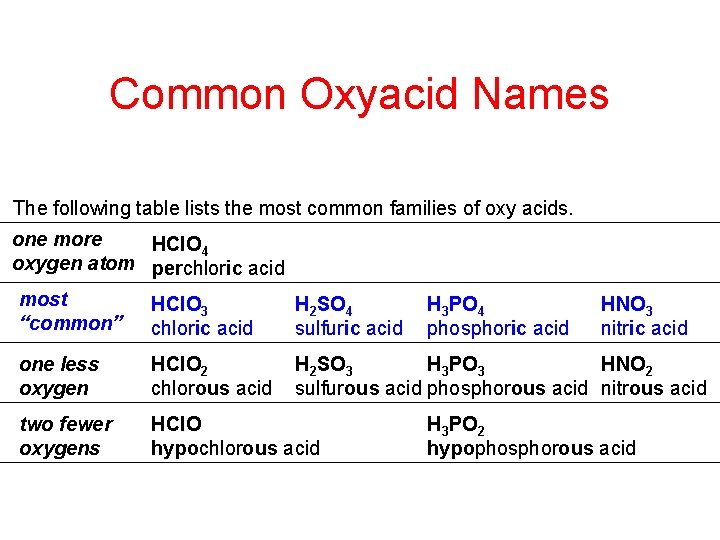
Common Oxyacid Names The following table lists the most common families of oxy acids. one more HCl. O 4 oxygen atom perchloric acid most “common” HCl. O 3 chloric acid H 2 SO 4 sulfuric acid one less oxygen HCl. O 2 chlorous acid H 2 SO 3 H 3 PO 3 HNO 2 sulfurous acid phosphorous acid nitrous acid two fewer oxygens HCl. O hypochlorous acid H 3 PO 4 phosphoric acid HNO 3 nitric acid H 3 PO 2 hypophosphorous acid

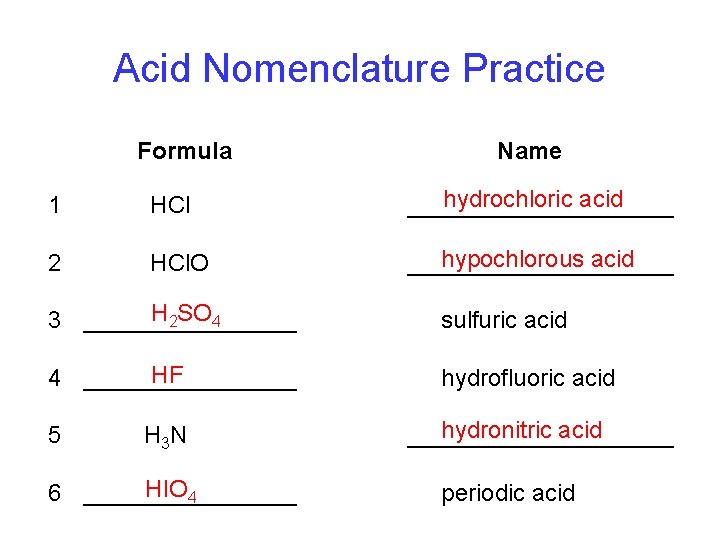
Acid Nomenclature Practice Formula Name 1 HCl hydrochloric acid __________ 2 HCl. O hypochlorous acid __________ H 2 SO 4 3 ________ sulfuric acid HF 4 ________ hydrofluoric acid 5 H 3 N HIO 4 6 ________ hydronitric acid __________ periodic acid

Covalent Compounds Nonmetal + Nonmetal

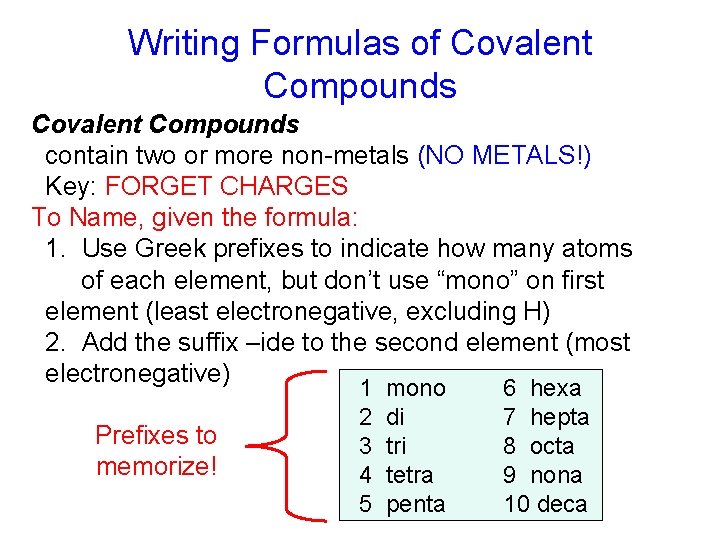
Writing Formulas of Covalent Compounds contain two or more non-metals (NO METALS!) Key: FORGET CHARGES To Name, given the formula: 1. Use Greek prefixes to indicate how many atoms of each element, but don’t use “mono” on first element (least electronegative, excluding H) 2. Add the suffix –ide to the second element (most electronegative) Prefixes to memorize! 1 2 3 4 5 mono di tri tetra penta 6 hexa 7 hepta 8 octa 9 nona 10 deca

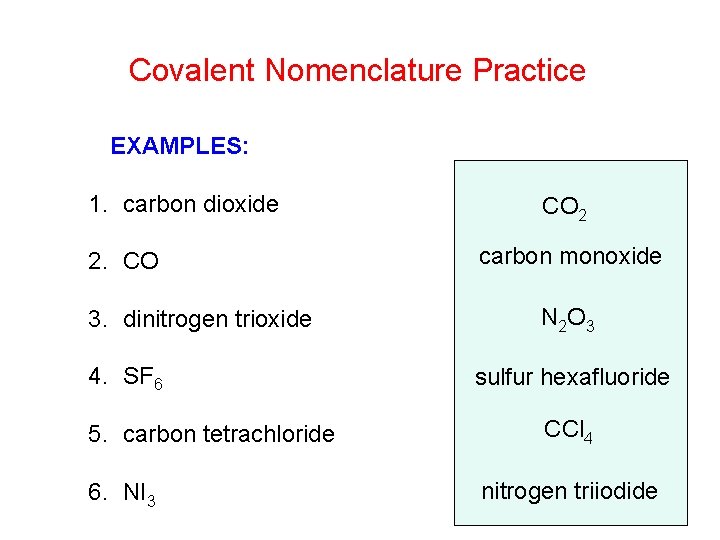
Covalent Nomenclature Practice EXAMPLES: 1. carbon dioxide 2. CO 3. dinitrogen trioxide 4. SF 6 5. carbon tetrachloride 6. NI 3 CO 2 carbon monoxide N 2 O 3 sulfur hexafluoride CCl 4 nitrogen triiodide

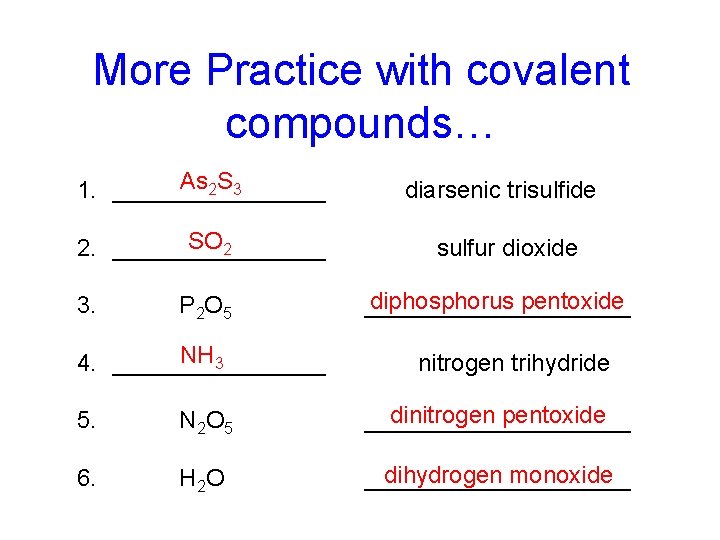
More Practice with covalent compounds… As 2 S 3 1. ________ diarsenic trisulfide SO 2 2. ________ sulfur dioxide 3. P 2 O 5 NH 3 4. ________ diphosphorus pentoxide __________ nitrogen trihydride 5. N 2 O 5 dinitrogen pentoxide __________ 6. H 2 O dihydrogen monoxide __________

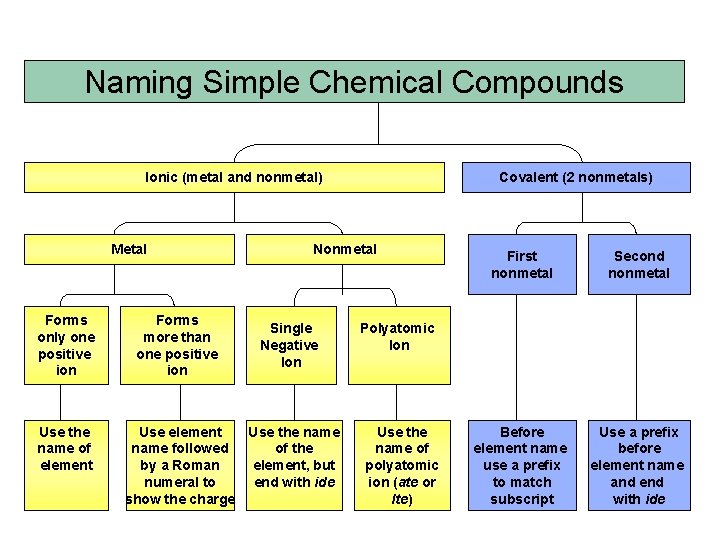
Naming Simple Chemical Compounds Ionic (metal and nonmetal) Metal Forms only one positive ion Use the name of element Forms more than one positive ion Covalent (2 nonmetals) Nonmetal Single Negative Ion Use element Use the name followed of the by a Roman element, but numeral to end with ide show the charge First nonmetal Second nonmetal Before element name use a prefix to match subscript Use a prefix before element name and end with ide Polyatomic Ion Use the name of polyatomic ion (ate or Ite)

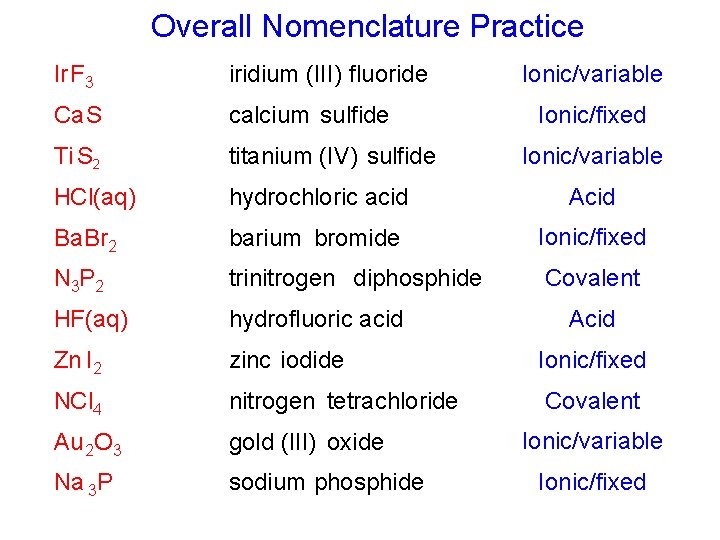
Overall Nomenclature Practice Ionic/variable Ir F 3 iridium (III) fluoride Ca S calcium sulfide Ti S 2 titanium (IV) sulfide HCl(aq) hydrochloric acid Acid Ba. Br 2 barium bromide Ionic/fixed N 3 P 2 trinitrogen diphosphide Covalent HF(aq) hydrofluoric acid Zn I 2 zinc iodide Ionic/fixed NCl 4 nitrogen tetrachloride Covalent Au 2 O 3 gold (III) oxide Na 3 P sodium phosphide Ionic/fixed Ionic/variable Acid Ionic/variable Ionic/fixed

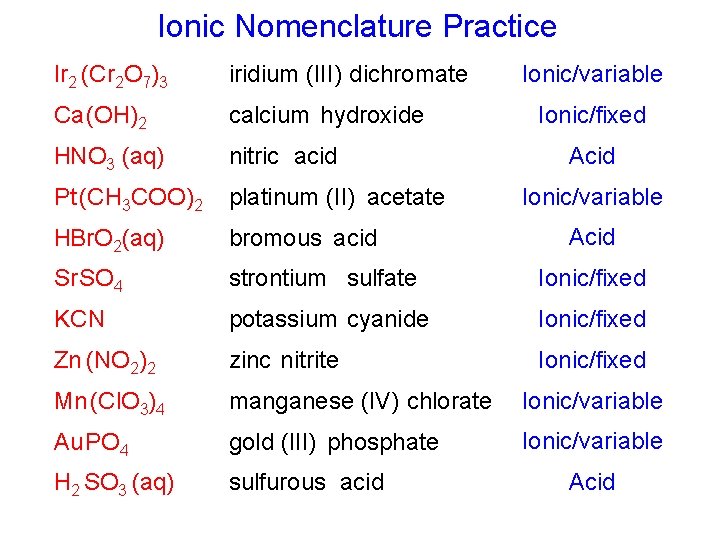
Ionic Nomenclature Practice Ionic/variable Ir 2 (Cr 2 O 7)3 iridium (III) dichromate Ca (OH)2 calcium hydroxide HNO 3 (aq) nitric acid Pt (CH 3 COO)2 platinum (II) acetate HBr. O 2(aq) bromous acid Sr SO 4 strontium sulfate Ionic/fixed KCN potassium cyanide Ionic/fixed Zn (NO 2)2 zinc nitrite Ionic/fixed Mn (Cl. O 3)4 manganese (IV) chlorate Ionic/variable Au PO 4 gold (III) phosphate Ionic/variable H 2 SO 3 (aq) sulfurous acid Ionic/fixed Acid Ionic/variable Acid

Covalent Ionic (M + NM) Two or more NM’s Variable charge cation carbon sulfur. N tetrabromide dichloride NCl O 35 2 vanadium niobium. Mn Pt(IO (V) (II) perchlorate )4 2 S 53 chromate Greek prefixes Charge Criss-Cross Rule Roman numeral Ionic (M + NM) Fixed charge cation rubidium sulfate NH 4 KI Cl. O barium oxide 3 Roman numeral for name only Polyatomic ions OK Where would you file these? VCr. O 4 dinitrogen pentoxide Ba. O platinum (IV) iodate CBr 4 ammonium chlorate Nb(Cl. O 4)5 potassium iodide SCl 2 nitrogen trichloride Rb 2 SO 4 manganese (V) sulfide

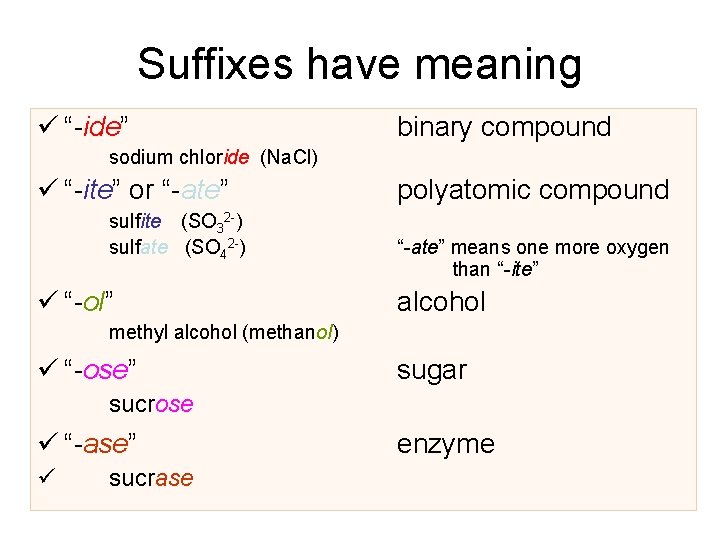
Suffixes have meaning ü “-ide” binary compound sodium chloride (Na. Cl) ü “-ite” or “-ate” sulfite (SO 32 -) sulfate (SO 42 -) ü “-ol” polyatomic compound “-ate” means one more oxygen than “-ite” alcohol methyl alcohol (methanol) ü “-ose” sugar sucrose ü “-ase” ü sucrase enzyme

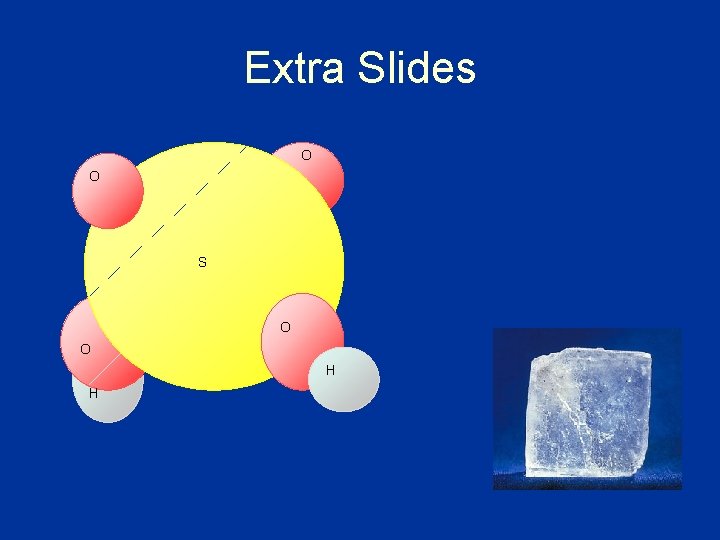
Extra Slides O O S O O H H

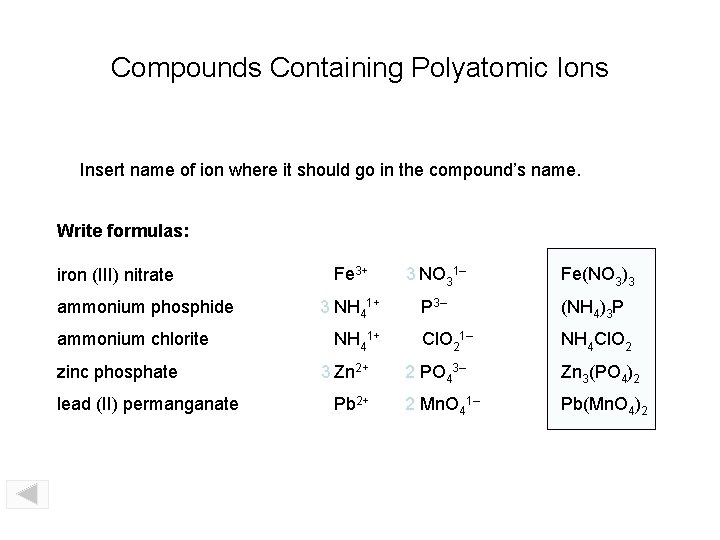
Compounds Containing Polyatomic Ions Insert name of ion where it should go in the compound’s name. Write formulas: iron (III) nitrate ammonium phosphide ammonium chlorite zinc phosphate lead (II) permanganate Fe 3+ 3 NO 31– Fe(NO 3)3 3 NH 41+ P 3– (NH 4)3 P Cl. O 21– NH 4 Cl. O 2 NH 41+ 3 Zn 2+ Pb 2+ 2 PO 43– Zn 3(PO 4)2 2 Mn. O 41– Pb(Mn. O 4)2

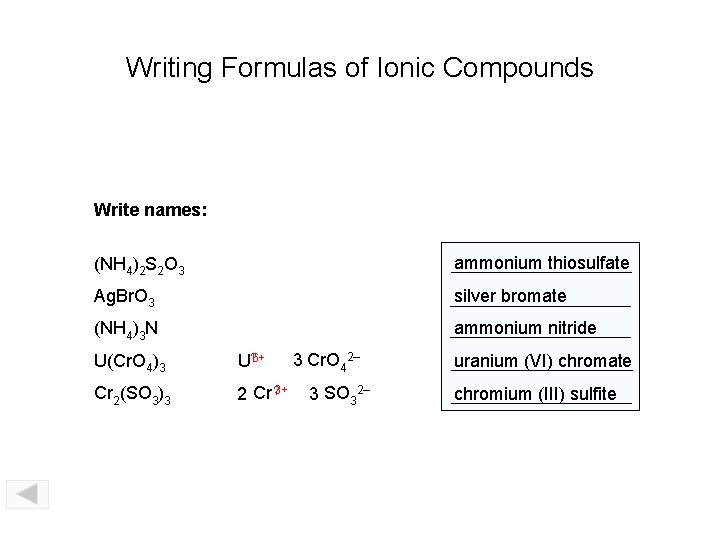
Writing Formulas of Ionic Compounds Write names: (NH 4)2 S 2 O 3 ammonium thiosulfate Ag. Br. O 3 silver bromate (NH 4)3 N ammonium nitride U(Cr. O 4)3 U? 6+ Cr 2(SO 3)3 2 Cr ? 3+ 3 Cr. O 42– 3 SO 32– uranium (VI) chromate chromium (III) sulfite

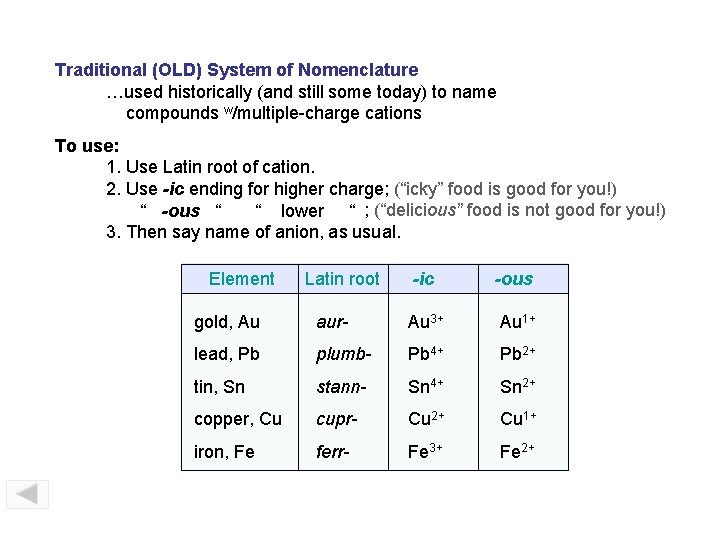
Traditional (OLD) System of Nomenclature …used historically (and still some today) to name compounds w/multiple-charge cations To use: 1. Use Latin root of cation. 2. Use -ic ending for higher charge; (“icky” food is good for you!) “ -ous “ “ lower “ ; (“delicious” food is not good for you!) 3. Then say name of anion, as usual. Element Latin root -ic -ous gold, Au aur- Au 3+ Au 1+ lead, Pb plumb- Pb 4+ Pb 2+ tin, Sn stann- Sn 4+ Sn 2+ copper, Cu cupr- Cu 2+ Cu 1+ iron, Fe ferr- Fe 3+ Fe 2+

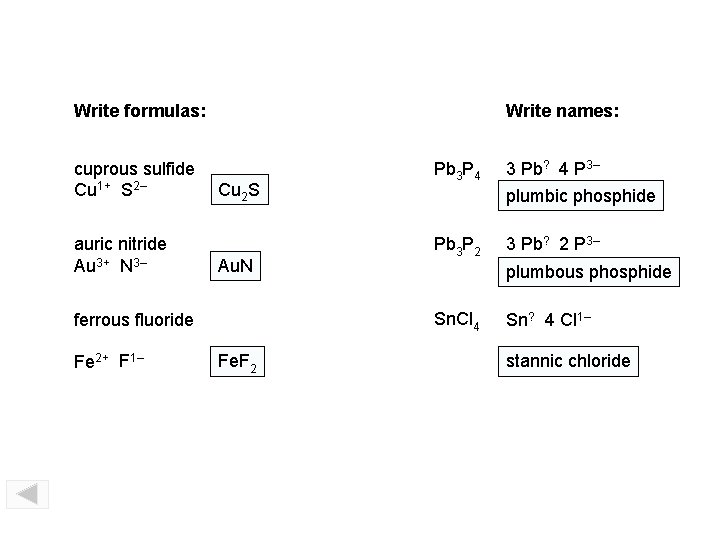
Write formulas: cuprous sulfide Cu 1+ S 2– auric nitride Au 3+ N 3– Write names: Cu 2 S Au. N 3 Pb? 4 P 3– plumbic phosphide Pb 3 P 2 3 Pb? 2 P 3– plumbous phosphide Sn. Cl 4 ferrous fluoride Fe 2+ F 1– Pb 3 P 4 Fe. F 2 Sn? 4 Cl 1– stannic chloride

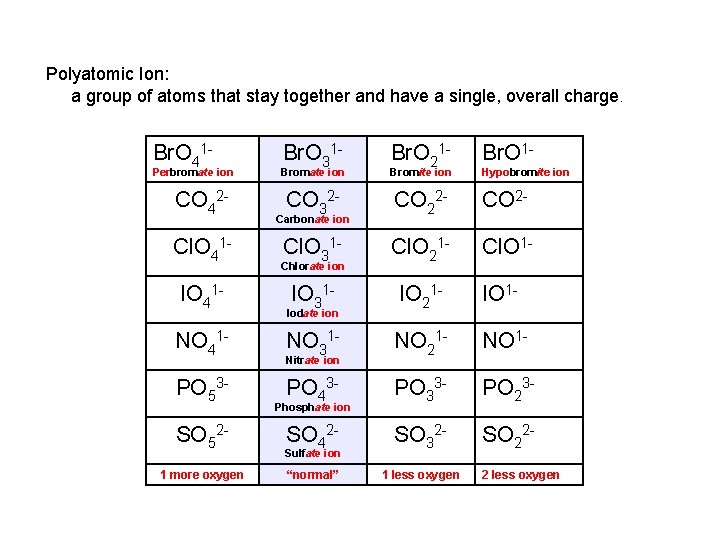
Polyatomic Ion: a group of atoms that stay together and have a single, overall charge. Br. O 41 - Perbromate ion CO 42 Cl. O 41 IO 41 NO 41 PO 53 SO 521 more oxygen Br. O 31 - Br. O 1 - Bromate ion Br. O 21 - Bromite ion CO 32 - CO 2 - Cl. O 31 - Cl. O 21 - Cl. O 1 - IO 31 - IO 21 - IO 1 - NO 31 - NO 21 - NO 1 - PO 43 - PO 33 - PO 23 - SO 42 - SO 32 - SO 22 - “normal” 1 less oxygen Carbonate ion Chlorate ion Iodate ion Nitrate ion Phosphate ion Sulfate ion Hypobromite ion 2 less oxygen

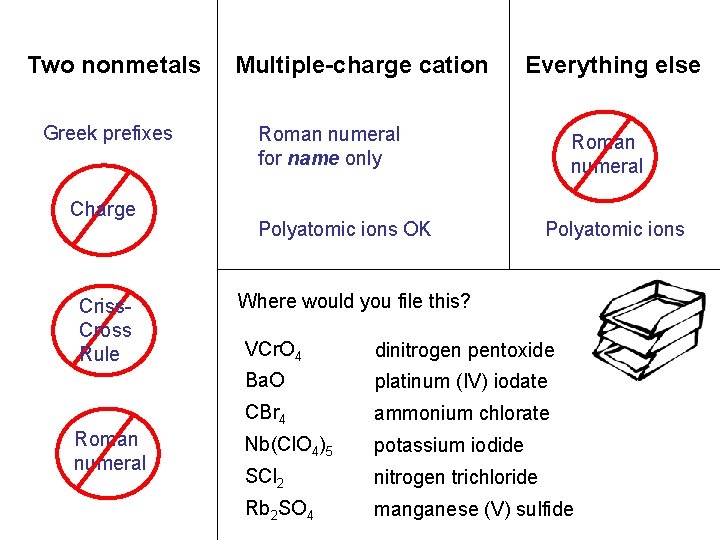
Two nonmetals Multiple-charge cation Everything else carbon sulfur. N tetrabromide dichloride NCl O 35 2 vanadium niobium. Mn Pt(IO (V) (II) perchlorate )4 2 S 53 chromate rubidium sulfate NH 4 KI Cl. O barium oxide 3 Greek prefixes Charge Criss. Cross Rule Roman numeral for name only Polyatomic ions OK Roman numeral Polyatomic ions OK Where would you file this? VCr. O 4 dinitrogen pentoxide Ba. O platinum (IV) iodate CBr 4 ammonium chlorate Nb(Cl. O 4)5 potassium iodide SCl 2 nitrogen trichloride Rb 2 SO 4 manganese (V) sulfide

Two nonmetals Greek prefixes Charge Criss. Cross Rule Roman numeral Multiple-charge cation Everything else Roman numeral for name only Polyatomic ions OK Roman numeral Polyatomic ions Where would you file this? VCr. O 4 dinitrogen pentoxide Ba. O platinum (IV) iodate CBr 4 ammonium chlorate Nb(Cl. O 4)5 potassium iodide SCl 2 nitrogen trichloride Rb 2 SO 4 manganese (V) sulfide

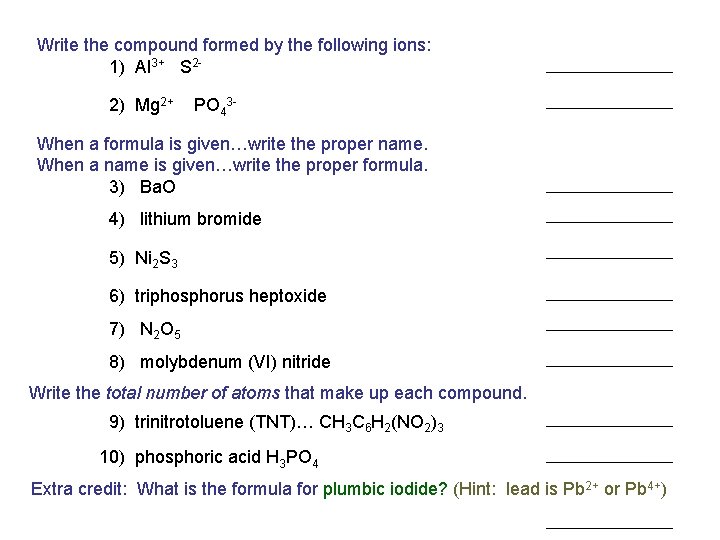
Write the compound formed by the following ions: 1) Al 3+ S 22) Mg 2+ PO 43 - When a formula is given…write the proper name. When a name is given…write the proper formula. 3) Ba. O 4) lithium bromide 5) Ni 2 S 3 6) triphosphorus heptoxide 7) N 2 O 5 8) molybdenum (VI) nitride Write the total number of atoms that make up each compound. 9) trinitrotoluene (TNT)… CH 3 C 6 H 2(NO 2)3 10) phosphoric acid H 3 PO 4 Extra credit: What is the formula for plumbic iodide? (Hint: lead is Pb 2+ or Pb 4+)

Write the compound formed by the following ions: 1) Al 3+ S 22) Mg 2+ PO 43 - When a formula is given…write the proper name. When a name is given…write the proper formula. 3) Ba. O POP QUIZ 4) lithium bromide 5) Ni 2 S 3 6) triphosphorus heptoxide 7) N 2 O 5 8) molybdenum (VI) nitride Write the total number of atoms that make up each compound. 9) trinitrotoluene (TNT)… CH 3 C 6 H 2(NO 2)3 10) phosphoric acid H 3 PO 4 Extra credit: What is the formula for plumbic iodide? (Hint: lead is Pb 2+ or Pb 4+)