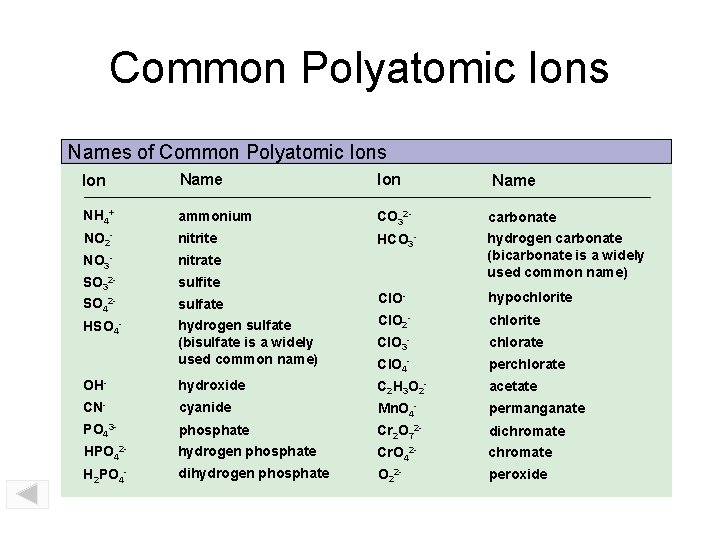
Common Polyatomic Ions Names of Common Polyatomic Ions
- Slides: 4

Common Polyatomic Ions Names of Common Polyatomic Ions Ion Name NH 4+ ammonium CO 32 - carbonate NO 2 - nitrite HCO 3 - NO 3 - nitrate SO 32 - sulfite hydrogen carbonate (bicarbonate is a widely used common name) SO 42 - sulfate Cl. O- hypochlorite HSO 4 - hydrogen sulfate (bisulfate is a widely used common name) Cl. O 2 - chlorite Cl. O 3 - chlorate Cl. O 4 - perchlorate OH- hydroxide C 2 H 3 O 2 - acetate CN- cyanide Mn. O 4 - permanganate PO 43 - phosphate Cr 2 O 72 - dichromate HPO 42 - hydrogen phosphate Cr. O 42 - chromate H 2 PO 4 - dihydrogen phosphate O 22 - peroxide

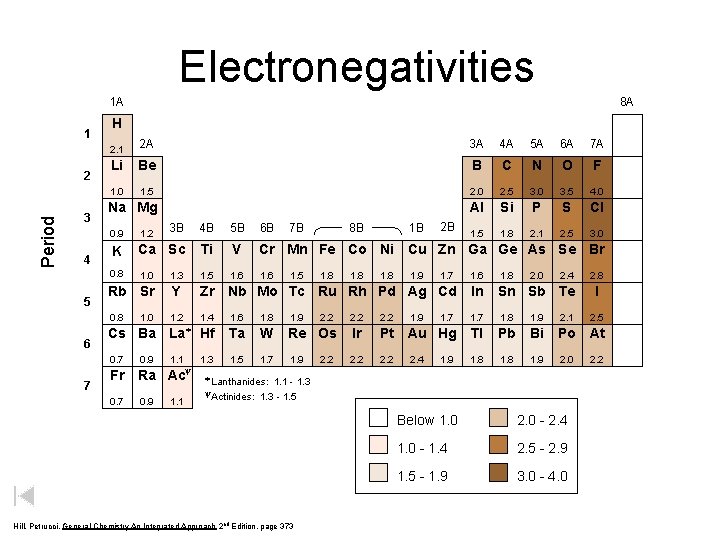
Electronegativities 1 A 1 Period 2 3 4 5 6 7 8 A H 2. 1 2 A 3 A 4 A 5 A 6 A 7 A Li Be B C N O F 1. 0 1. 5 2. 0 2. 5 3. 0 3. 5 4. 0 Al Si P S Cl 1. 5 1. 8 2. 1 2. 5 3. 0 Na Mg 1. 2 3 B 4 B 5 B 6 B K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 0. 8 1. 0 1. 3 1. 5 1. 6 1. 7 1. 6 1. 8 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 0. 8 1. 2 1. 4 1. 6 1. 8 1. 9 2. 2 1. 7 1. 8 1. 9 2. 1 Cs Ba La* Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At 0. 7 1. 1 1. 3 1. 5 1. 7 1. 9 2. 2 1. 8 1. 9 2. 0 Fr 0. 7 1. 0 0. 9 y Ra Ac 0. 9 1. 1 8 B 7 B 1. 5 1. 8 2. 2 1. 8 1 B 2 B 0. 9 1. 8 1. 9 2. 4 1. 9 2. 0 2. 4 * Lanthanides: 1. 1 - 1. 3 - 1. 5 y. Actinides: Hill, Petrucci, General Chemistry An Integrated Approach 2 nd Edition, page 373 Below 1. 0 2. 0 - 2. 4 1. 0 - 1. 4 2. 5 - 2. 9 1. 5 - 1. 9 3. 0 - 4. 0 2. 8 I 2. 5 2. 2

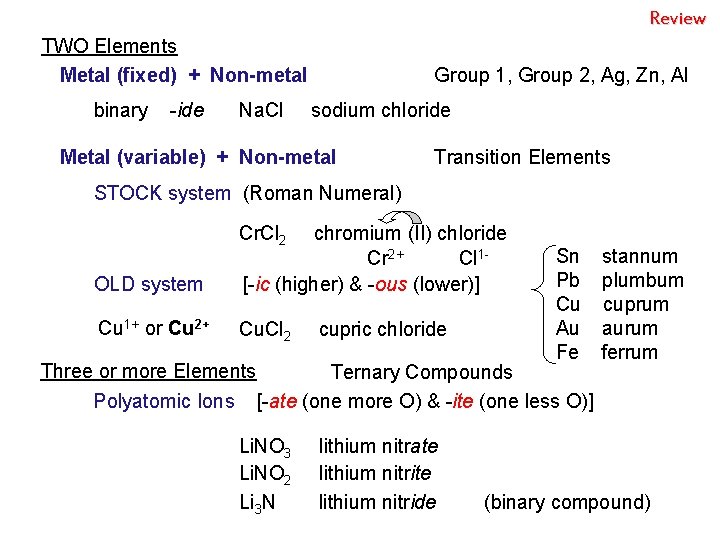
Review TWO Elements Metal (fixed) + Non-metal binary -ide Na. Cl Group 1, Group 2, Ag, Zn, Al sodium chloride Metal (variable) + Non-metal Transition Elements STOCK system (Roman Numeral) Cr. Cl 2 OLD system chromium (II) chloride Cr 2+ Cl 1[-ic (higher) & -ous (lower)] Cu 1+ or Cu 2+ Cu. Cl 2 cupric chloride Sn Pb Cu Au Fe Three or more Elements Ternary Compounds Polyatomic Ions [-ate (one more O) & -ite (one less O)] Li. NO 3 Li. NO 2 Li 3 N lithium nitrate lithium nitride stannum plumbum cuprum aurum ferrum (binary compound)

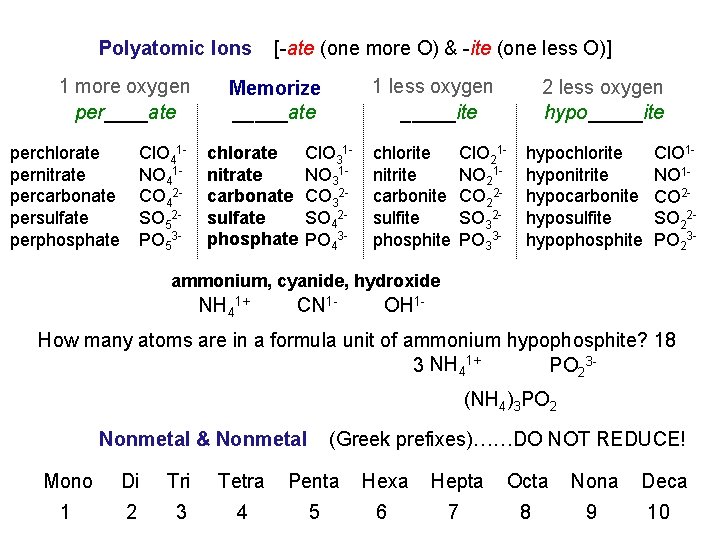
Polyatomic Ions 1 more oxygen per____ate perchlorate pernitrate percarbonate persulfate perphosphate Cl. O 41 NO 41 CO 42 SO 52 PO 53 - [-ate (one more O) & -ite (one less O)] 1 less oxygen _____ite Memorize NORMAL _____ate chlorate nitrate carbonate sulfate phosphate Cl. O 31 NO 31 CO 32 SO 42 PO 43 - chlorite nitrite carbonite sulfite phosphite 2 less oxygen hypo_____ite Cl. O 21 NO 21 CO 22 SO 32 PO 33 - hypochlorite hyponitrite hypocarbonite hyposulfite hypophosphite Cl. O 1 NO 1 CO 2 SO 22 PO 23 - ammonium, cyanide, hydroxide NH 41+ CN 1 - OH 1 - How many atoms are in a formula unit of ammonium hypophosphite? 18 3 NH 41+ PO 23(NH 4)3 PO 2 Nonmetal & Nonmetal (Greek prefixes)……DO NOT REDUCE! Mono Di Tri Tetra Penta Hexa Hepta Octa Nona Deca 1 2 3 4 5 6 7 8 9 10