COMMON PHARMACOLOGY PHARMACOKINETICS Barriers oral administration barriers of

COMMON PHARMACOLOGY PHARMACOKINETICS
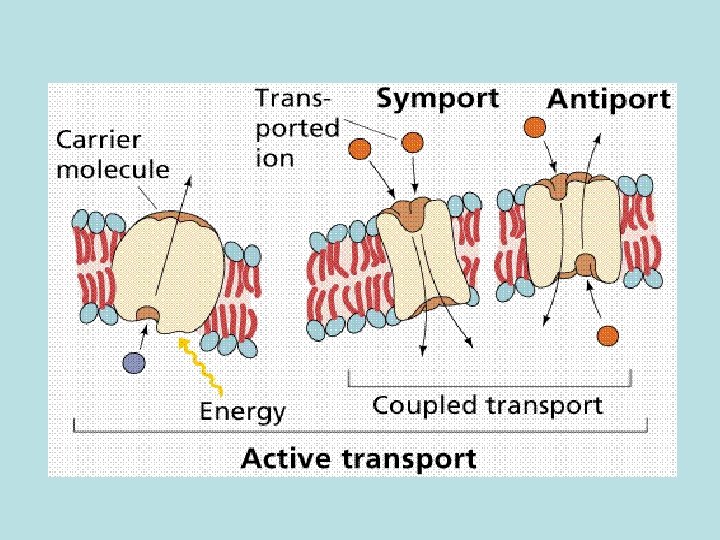
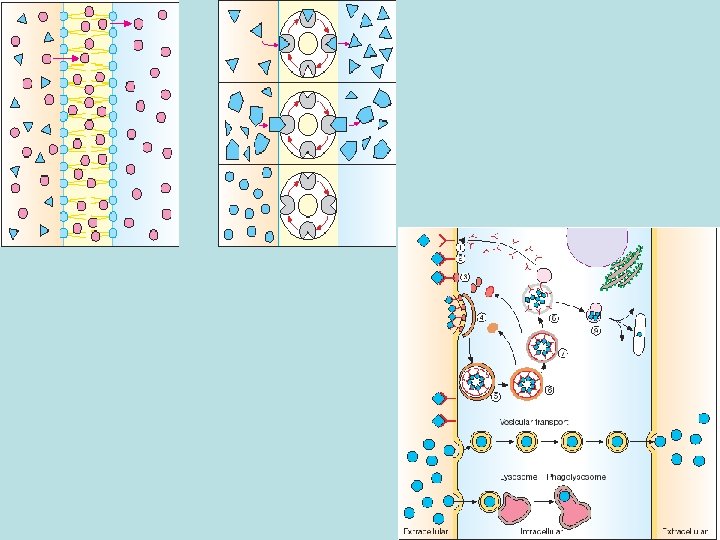
• Barriers: oral administration - barriers of GI mucous membrane cells and endothelial cells of the vessels; intravenous administration – layer of endothelial cells Main principles of drugs penetration through membranes • epithelium in biological small intestine is well penetrable – it contains multiple channels (canalicules) through which molecules with relatively low molecular weight (most of drugs) can penetrate; stomach has no canalicules – strong epithelium • penetration of drugs through cellular membranes - universal mechanisms (role in absorption, distribution, excretion): passive diffusion, facilitated diffusion (with a help of special carriers), filtration, active transport and pinocytosis
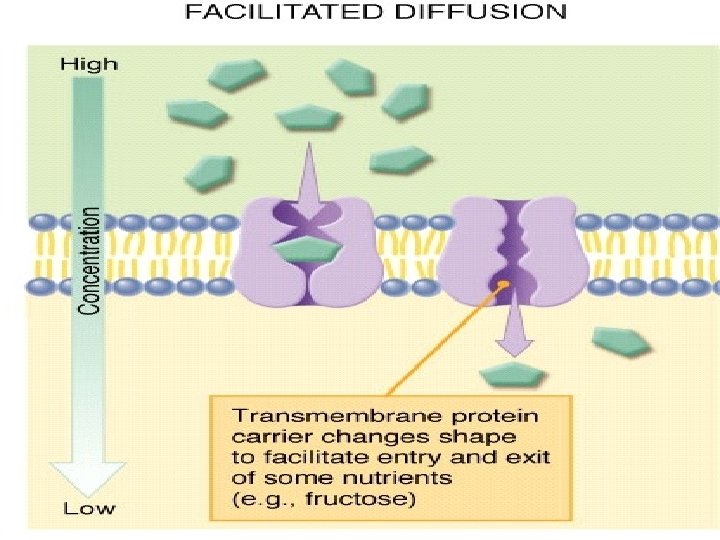
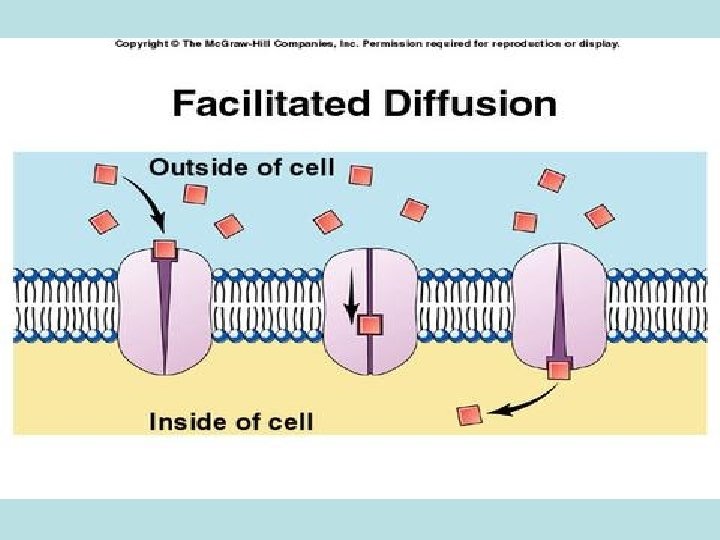
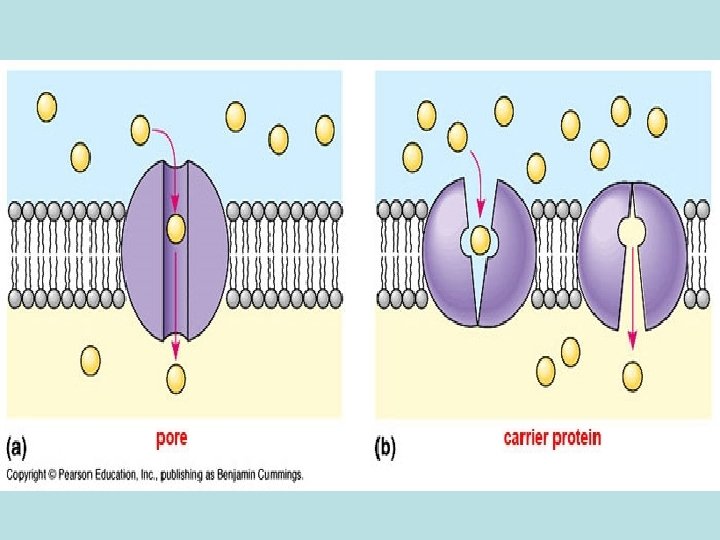
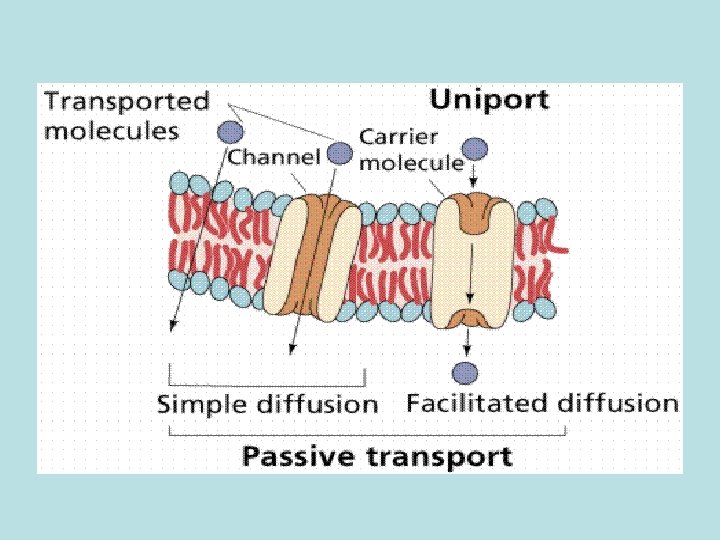
• Passive diffusion – along with gradient of concentration (from the zone with higher concentration to the zone with lower concentration ; it doesn’t need any energy and lasts until concentration of the substance from both sides of the membrane becomes equal): acetylsalicylic acid, aminazin, quinine, ether • Facilitated diffusion – with the help of proteins-transporters: glucose, aminoacids, vitamins (specific gastromucoprotein synthesized in stomach is necessary for absorption of vitamin В 12 in small intestine) • Filtration – through pores in membrane, the size of which is around 0, 35 -0, 8 nm. Substances with low molecular weight (water, urea etc. ) penetrate through pores. Ionized particles (cations, anions) practically do not penetrate through pores (the reason is заряд on the cell membrane) • Active transport – is provided by specific transport systems of the cells and needs energy since happens against gradient of concentration: cardiac glycosides, glucocortecosteroids, input of iodine into thyroid gland • Pinocytosis – in the place of drug molecule contact cell membrane invagination with formation of a vesicle happens, this vesicle plunges into cell: proteins, nucleonic acids, fat soluble vitamins
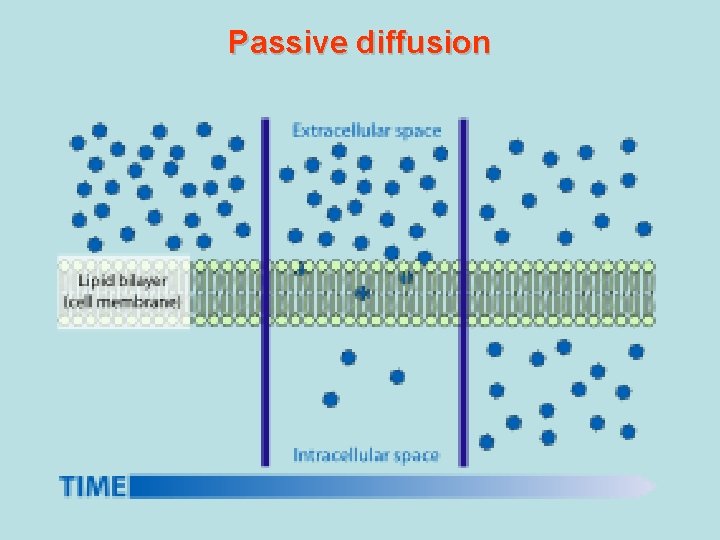
Passive diffusion
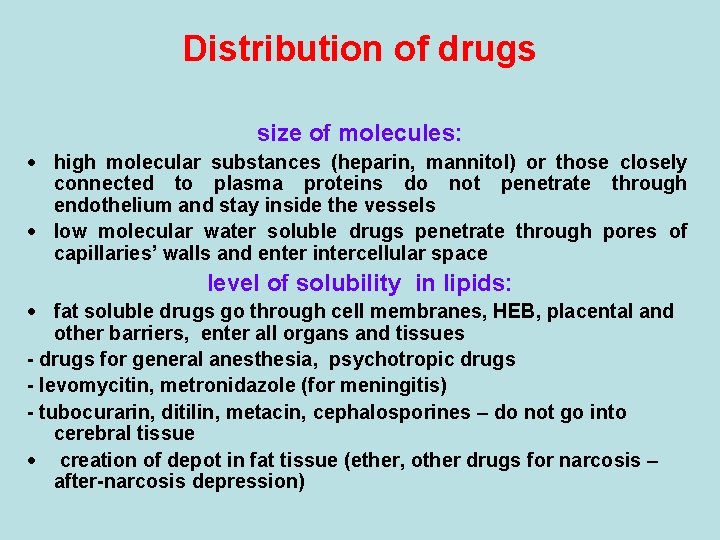
Distribution of drugs size of molecules: · high molecular substances (heparin, mannitol) or those closely connected to plasma proteins do not penetrate through endothelium and stay inside the vessels · low molecular water soluble drugs penetrate through pores of capillaries’ walls and enter intercellular space level of solubility in lipids: · fat soluble drugs go through cell membranes, HEB, placental and other barriers, enter all organs and tissues - drugs for general anesthesia, psychotropic drugs - levomycitin, metronidazole (for meningitis) - tubocurarin, ditilin, metacin, cephalosporines – do not go into cerebral tissue · creation of depot in fat tissue (ether, other drugs for narcosis – after-narcosis depression)
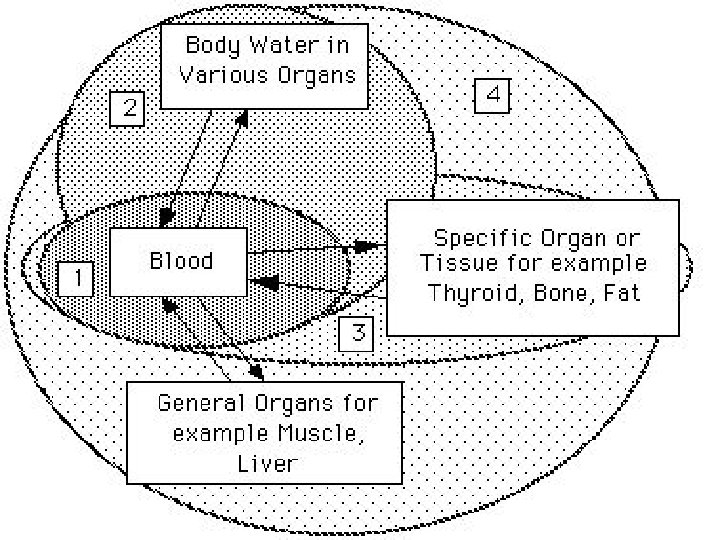
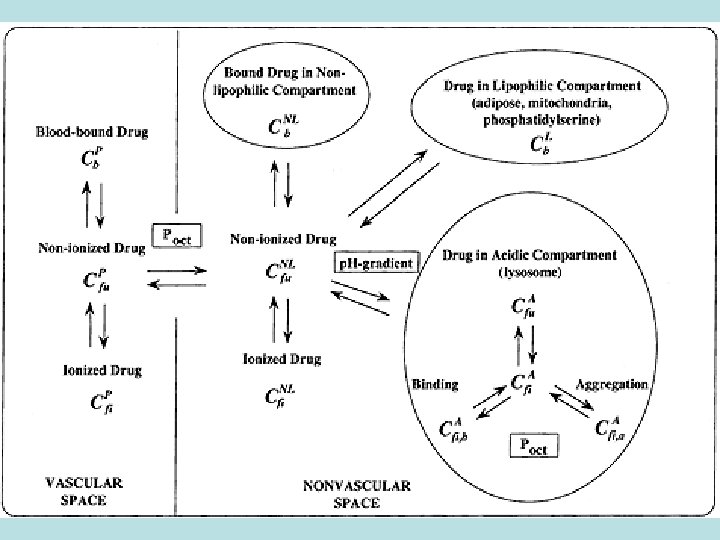
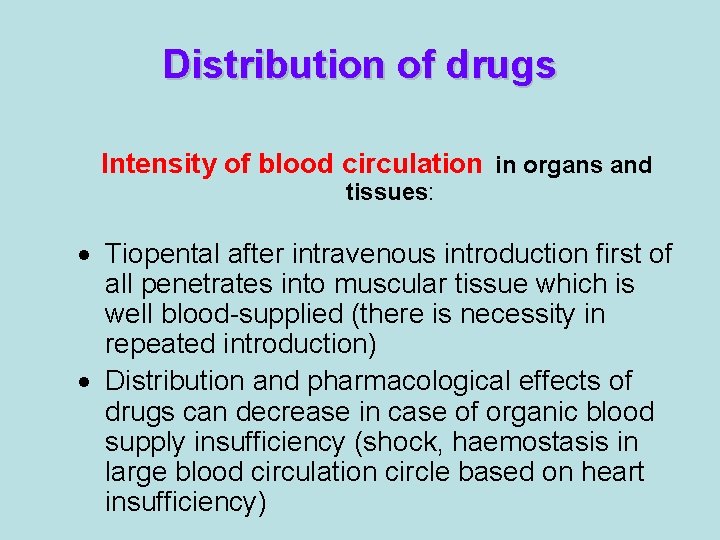
Distribution of drugs Intensity of blood circulation in organs and tissues: · Tiopental after intravenous introduction first of all penetrates into muscular tissue which is well blood-supplied (there is necessity in repeated introduction) · Distribution and pharmacological effects of drugs can decrease in case of organic blood supply insufficiency (shock, haemostasis in large blood circulation circle based on heart insufficiency)
CONNECTION OF DRUGS WITH BLOOD PLASMA PROTEINS • Albumin, lipoproteins, 1 -acid gycoprotein and globulins • specific proteins-carriers: glucocorticosteroids – transcortin, vitamin В 12 – transcobalamin, iron ions – transferrin, copper ions – ceruloplasmin free and bound with proteins forms of a drug stay in condition of dynamic balance drug bound with plasma proteins is pharmacologically inactive !!!

• in case of hypoalbuminemia (liver diseases, burn disease, protein starvation, elderly): increasing of free fraction of a drug, increasing of pharmacological activity, development of toxic effects • high level of connection of blood proteins: diazepam, butamid, difenin, indometacin, furosemid, quinidine • competition for binding with plasma proteins: sodium valproate forces out difenin – increasing of free fraction of the last - toxic effects • high level of sulfadimetoxin, sulfapirydasin binding with blood proteins causes prolongation of their action
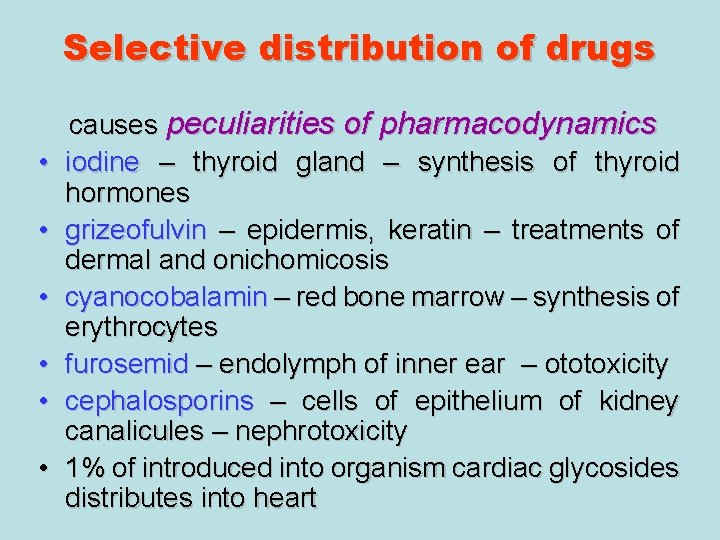
Selective distribution of drugs • • • causes peculiarities of pharmacodynamics iodine – thyroid gland – synthesis of thyroid hormones grizeofulvin – epidermis, keratin – treatments of dermal and onichomicosis cyanocobalamin – red bone marrow – synthesis of erythrocytes furosemid – endolymph of inner ear – ototoxicity cephalosporins – cells of epithelium of kidney canalicules – nephrotoxicity 1% of introduced into organism cardiac glycosides distributes into heart
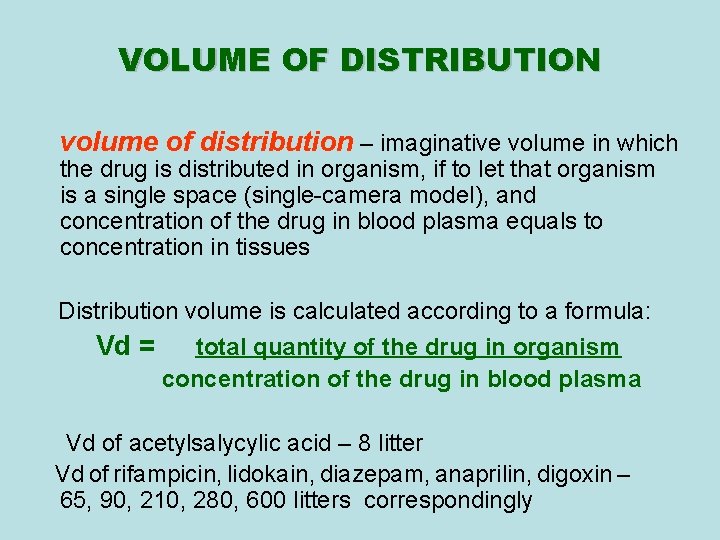
VOLUME OF DISTRIBUTION volume of distribution – imaginative volume in which the drug is distributed in organism, if to let that organism is a single space (single-camera model), and concentration of the drug in blood plasma equals to concentration in tissues Distribution volume is calculated according to a formula: Vd = total quantity of the drug in organism concentration of the drug in blood plasma Vd of acetylsalycylic acid – 8 litter Vd of rifampicin, lidokain, diazepam, anaprilin, digoxin – 65, 90, 210, 280, 600 litters correspondingly

Depositing of drugs • extra- and intracellular depot of drugs • in blood plasma and on the ways of their excretion from the organism • ethambutol – in erythrocytes • tetracyclins – in bone tissue, in teeth • ampicillin, biseptol, nitroxolin, nalidixic acid – in kidneys • drugs for general anesthesia – in fat tissue

Metabolism of drugs Metabolism or biotransformation complex of processes which provide decreasing of toxicity and accelerate excreting of the molecule of a drug or other foreign substance after its incoming into the organism
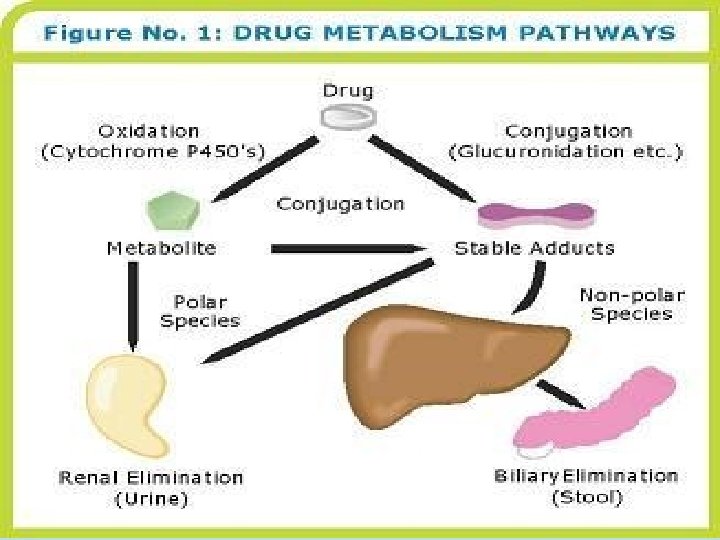
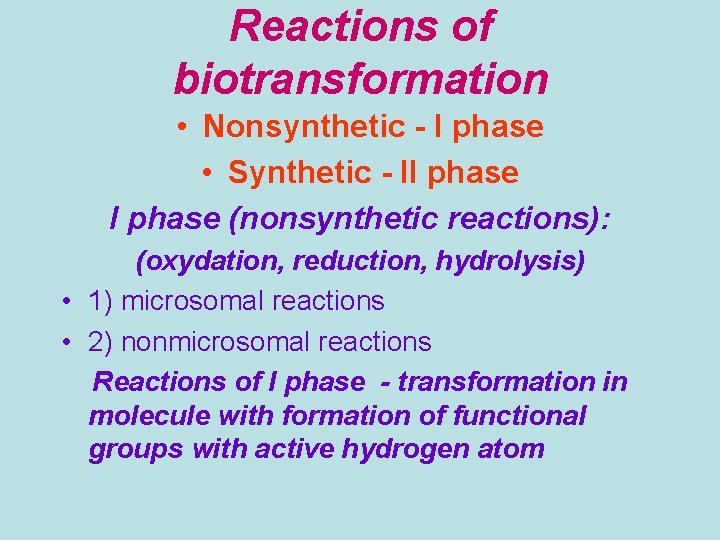
Reactions of biotransformation • Nonsynthetic - І phase • Synthetic - ІІ phase (nonsynthetic reactions): (oxydation, reduction, hydrolysis) • 1) microsomal reactions • 2) nonmicrosomal reactions Reactions of І phase - transformation in molecule with formation of functional groups with active hydrogen atom

ORGANS OF DRUGS METABOLISM • liver • • • kidneys muscle tissue intestinal wall lungs skin blood

Microsomal enzyme system Oxydoreductases, esterases, enzymes of proteins, lipids, glycerophosphatides, lipo- and glycoproteids, bile acids, cholesterol, prostaglandins biosynthesis, enzyme systems of biosynthesis of couple compounds, ethers of glucuronic and sulfur acids
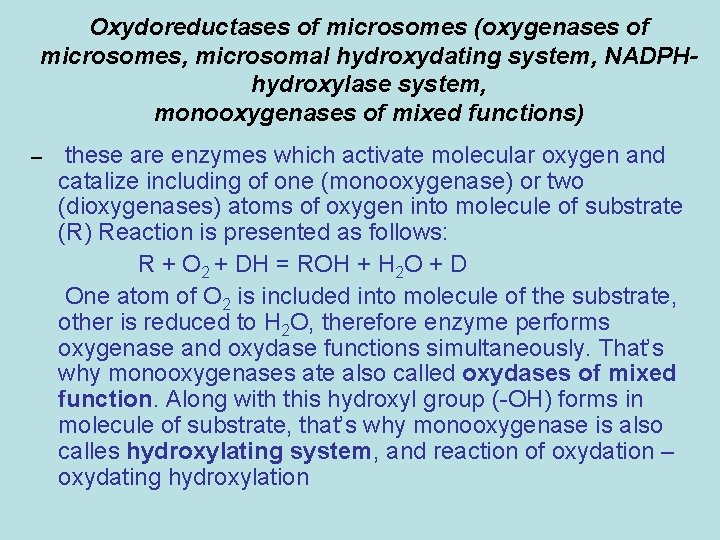
Oxydoreductases of microsomes (oxygenases of microsomes, microsomal hydroxydating system, NADPHhydroxylase system, monooxygenases of mixed functions) – these are enzymes which activate molecular oxygen and catalize including of one (monooxygenase) or two (dioxygenases) atoms of oxygen into molecule of substrate (R) Reaction is presented as follows: R + O 2 + DН = ROH + H 2 O + D One atom of О 2 is included into molecule of the substrate, other is reduced to Н 2 О, therefore enzyme performs oxygenase and oxydase functions simultaneously. That’s why monooxygenases ate also called oxydases of mixed function. Along with this hydroxyl group (-ОН) forms in molecule of substrate, that’s why monooxygenase is also calles hydroxylating system, and reaction of oxydation – oxydating hydroxylation

CYP 450 CYP-450 – hemoprotein, which is able to interact with substrate of oxydation, to activate oxygen and combine it with substrate. Specifically on CYР-450 reactions of hydroxydation are performed large amount of isoforms of this enzyme – possibility of its binding with different substrates and taking part in their metabolism There are 24 isoforms of CYР-450 in microsomes of human liver Multiplicity of the enzyme has a group character: one isoform of CYР-450 interacts not only with one substrate but with a group of substances
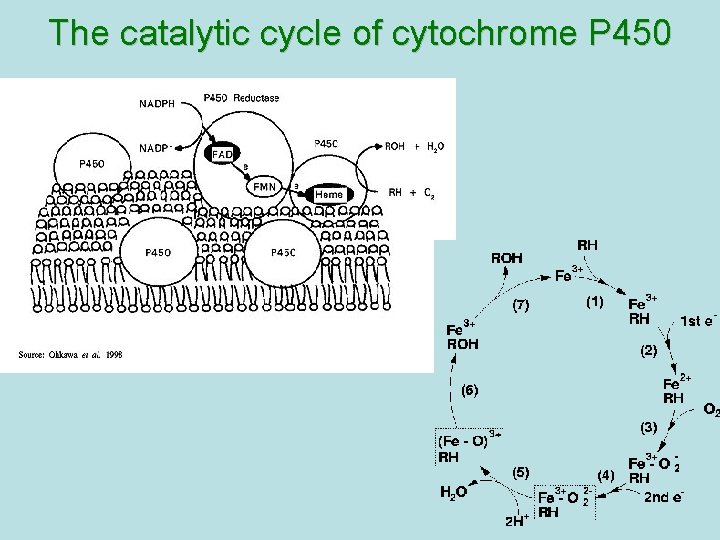
The catalytic cycle of cytochrome P 450
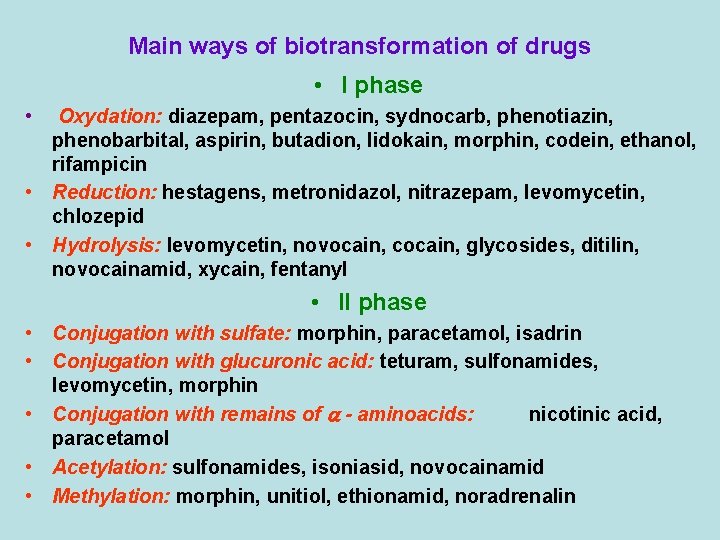
Main ways of biotransformation of drugs • I phase • Oxydation: diazepam, pentazocin, sydnocarb, phenotiazin, phenobarbital, aspirin, butadion, lidokain, morphin, codein, ethanol, rifampicin • Reduction: hestagens, metronidazol, nitrazepam, levomycetin, chlozepid • Hydrolysis: levomycetin, novocain, cocain, glycosides, ditilin, novocainamid, xycain, fentanyl • II phase • Conjugation with sulfate: morphin, paracetamol, isadrin • Conjugation with glucuronic acid: teturam, sulfonamides, levomycetin, morphin • Conjugation with remains of - aminoacids: nicotinic acid, paracetamol • Acetylation: sulfonamides, isoniasid, novocainamid • Methylation: morphin, unitiol, ethionamid, noradrenalin
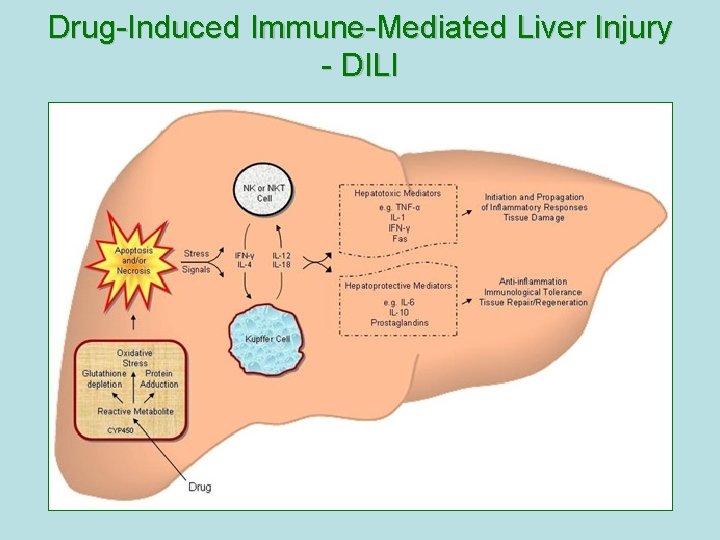
Drug-Induced Immune-Mediated Liver Injury - DILI

Metabolism in the intestinal wall • • Synthetic and nonsynthetic reactions take place Isadrin – conjugation with sulfate Hydrlalasin - acetylation Penicillin, aminazin – metabolism with nonspecific enzymes Methotrexat, levodopa – metabolism with intestinal bacteria

PRESYSTEMIC ELIMINATION presystemic elimination – extraction of the drug form blood circulatory system during it’s first going through the liver (first pass metabolism) – it leads to decreasing of bioavailability (and therefore, decreasing of biological activity) of drugs propranolol (anaprilin), labetolol, aminazin, acetylsalicylic acid, labetolol, hydralasin, isadrin, cortizone, lidokain, morphin, pentasocin, organic nitrates, reserpin
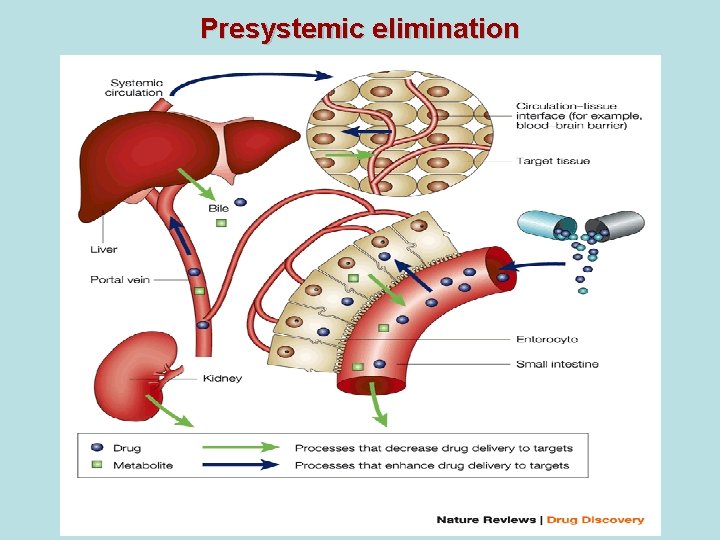
Presystemic elimination

Factors that influence on drug metabolism


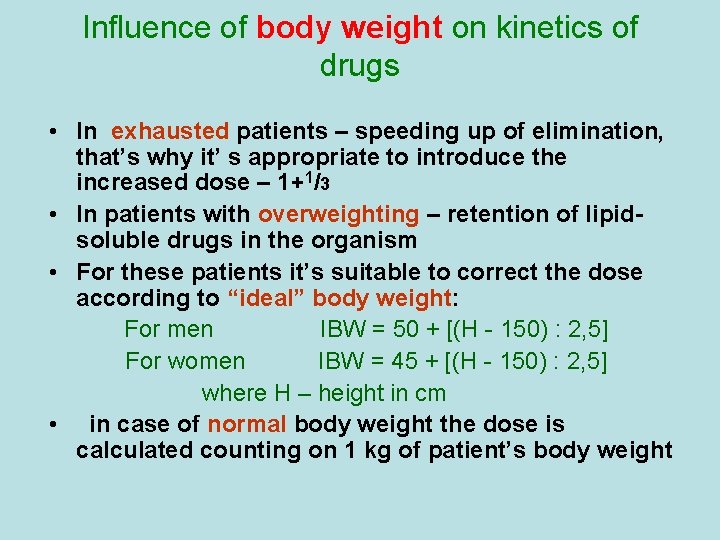
Influence of body weight on kinetics of drugs • In exhausted patients – speeding up of elimination, that’s why it’ s appropriate to introduce the increased dose – 1+1/3 • In patients with overweighting – retention of lipidsoluble drugs in the organism • For these patients it’s suitable to correct the dose according to “ideal” body weight: For men ІBW = 50 + [(Н - 150) : 2, 5] For women ІBW = 45 + [(Н - 150) : 2, 5] where Н – height in cm • in case of normal body weight the dose is calculated counting on 1 kg of patient’s body weight

Biotransformation of drugs into active (or more active) metabolites Initial drug • • • Allopurinol Amitriptilin Acetylsalicylic acid Butadion Diazepam Digitoxin Codein Cortizol Methyldopa Prednison Novocainamid Propranolol Active metabolite • • • Aloxantin Nortriptilin Salicylic acid Oxyfenbutazon Dismethyldiazepam Digoxin Morphine Hydrocortizon Methylnoradrenalin Prednisolon N-acetylnovocainamid N-oxypropranolol

Elimination of the drugs can be excreted in forms of metabolites or unchanged forms through different ways: kidneys, liver, lungs, intestines, sweat and mammary glands etc.
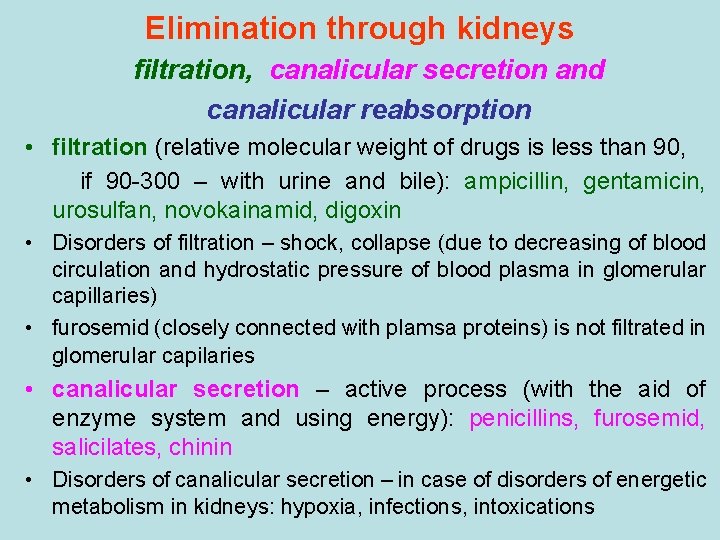
Elimination through kidneys filtration, canalicular secretion and canalicular reabsorption • filtration (relative molecular weight of drugs is less than 90, if 90 -300 – with urine and bile): ampicillin, gentamicin, urosulfan, novokainamid, digoxin • Disorders of filtration – shock, collapse (due to decreasing of blood circulation and hydrostatic pressure of blood plasma in glomerular capillaries) • furosemid (closely connected with plamsa proteins) is not filtrated in glomerular capilaries • canalicular secretion – active process (with the aid of enzyme system and using energy): penicillins, furosemid, salicilates, chinin • Disorders of canalicular secretion – in case of disorders of energetic metabolism in kidneys: hypoxia, infections, intoxications

Canalicular reabsorbtion (reversed absorbtion) lipid-soluble drugs are reabsorbed passively ionized drugs, which are weak acids or alkali are reabsorbed actively regulation of level of reabsorbtion - to speed up elimination of drugs – weak alkalis (antihistamine drugs, chinin, theophyllin) urine is made acidic (with ascorbinic acid, ammonium chloride) - to speed up elimination of drugs – weak acids (NSAID, including ASA, barbiturates, sulfonamides) urine is made alkaline (introduction of sodium hydrocarbonate)
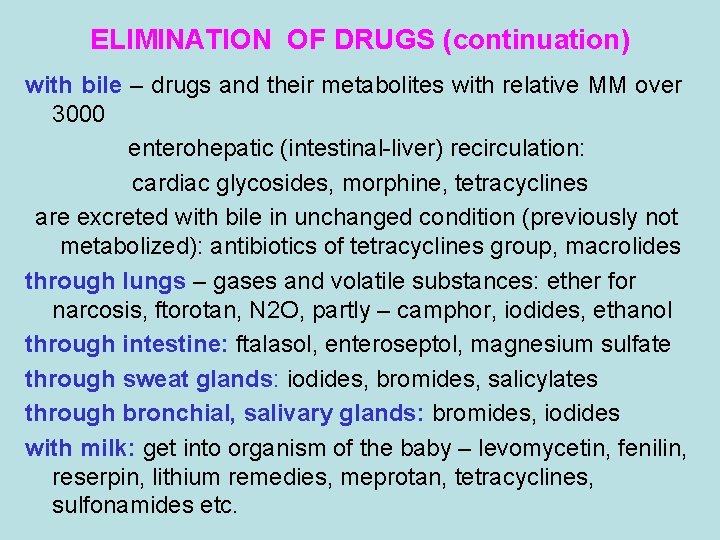
ELIMINATION OF DRUGS (continuation) with bile – drugs and their metabolites with relative MM over 3000 enterohepatic (intestinal-liver) recirculation: cardiac glycosides, morphine, tetracyclines are excreted with bile in unchanged condition (previously not metabolized): antibiotics of tetracyclines group, macrolides through lungs – gases and volatile substances: ether for narcosis, ftorotan, N 2 O, partly – camphor, iodides, ethanol through intestine: ftalasol, enteroseptol, magnesium sulfate through sweat glands: iodides, bromides, salicylates through bronchial, salivary glands: bromides, iodides with milk: get into organism of the baby – levomycetin, fenilin, reserpin, lithium remedies, meprotan, tetracyclines, sulfonamides etc.



GENERAL PHARMACOLOGY PHARMACODYNAMICS
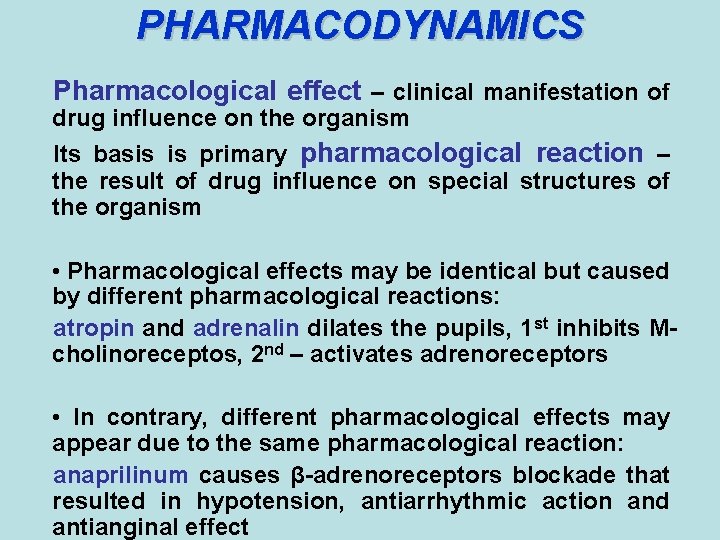
PHARMACODYNAMICS Pharmacological effect – clinical manifestation of drug influence on the organism Its basis is primary pharmacological reaction – the result of drug influence on special structures of the organism • Pharmacological effects may be identical but caused by different pharmacological reactions: atropin and adrenalin dilates the pupils, 1 st inhibits Mcholinoreceptos, 2 nd – activates adrenoreceptors • In contrary, different pharmacological effects may appear due to the same pharmacological reaction: anaprilinum causes β-adrenoreceptors blockade that resulted in hypotension, antiarrhythmic action and antianginal effect

PHARMACOLOGICAL EFFECTS • Local: astringent, covering, irritating, local anesthesia, necrotizing, adsorbing • Reflectory: as a result of local irritating (Sol. Ammonii caustici, Validolum, Charta Sinapis, expectorants of plant origin) • Resorbtive (systemic – after drug absorption or its introduction to blood): 1) direct (primary) and 2) indirect (secondary): cardiac glycosides: 1 – on heart, 2 – diuretic effect • Selective action (salbutamol, celecoxyb, doxazosin) • Nonspecific action – on all cells of the organism: drugs for general anesthesia, salts of heavy metals • Basic (beneficial) action an adverse reaction • Reversible and irreversible

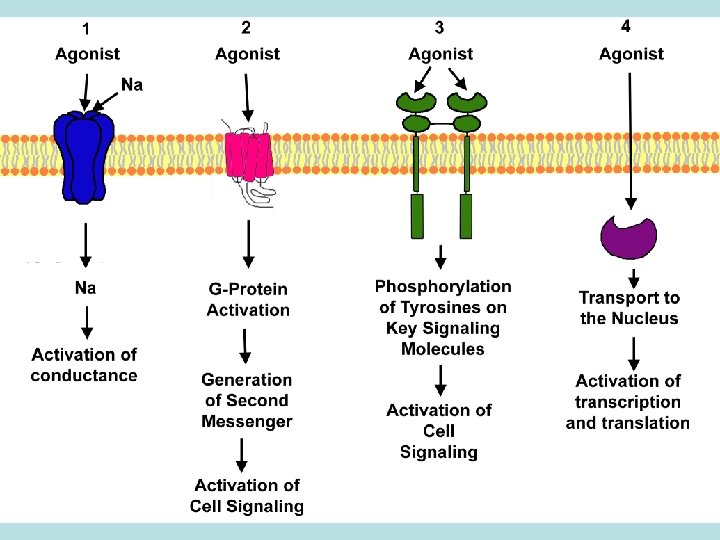
RECEPTOR THEORY OF DRUG ACTION Receptors – the places where drugs bind to tissues: macromolecules, enzymes, channels, transport systems, genes • Agonists: adrenalin, isadrine, morphine etc. • Antagonists: atropin, anaprilin, dimedrol etc. Конкурентні та неконкурентні антагоністи • Agonist-antagonist: labetolol ( 1, 1 -adrenoblocker, but activates 2 -adrenoreceptors), pentazocin (agonist delta- and kappa-opiate receptors and mu-receptors antagonist) •
RECEPTOR THEORY OF DRUG ACTION TYPES OF RECEPTORS Specific structures of cells: N- and M-cholinoreceptors, - і adrenoreceptors, dophamine-, opiate-, histamine-, serotonin-, receptors to angiotensin II, leucotryenic-, prostaglandine-, polypeptic and steroid hormones etc.
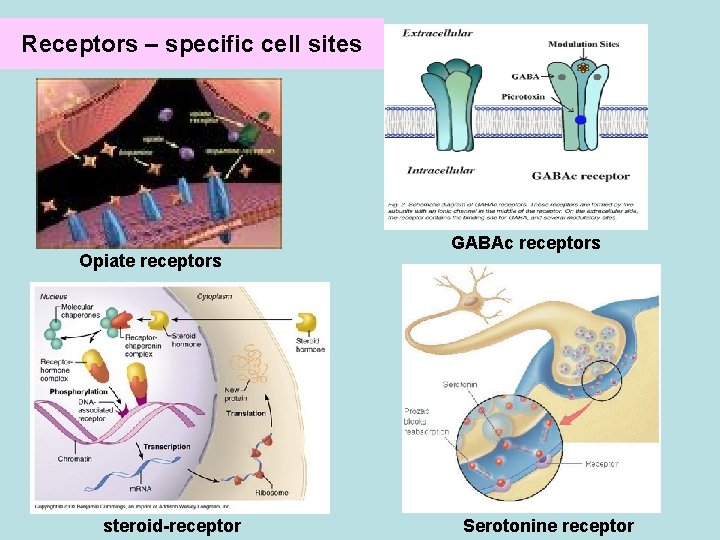
Receptors – specific cell sites Opiate receptors steroid-receptor GABAc receptors Serotonine receptor
TYPES OF RECEPTORS Receptors-enzymes: acethylcholinestherase (Proserine), monoaminoxydase in neurons of CNS (Nialamid), angiotensin converting enzyme (ACE-blockers – Captopril, Enalapril), K-, Na- ATPase (cardiac glycosides - Digoxin), H-, K-ATPase (proton pump) (Omeprasol), COG-1, COG-2 (nonsteroidal antiinflammatory agents – Diclofenac, Indometacin, Piroxycam, Meloxicam etc. )
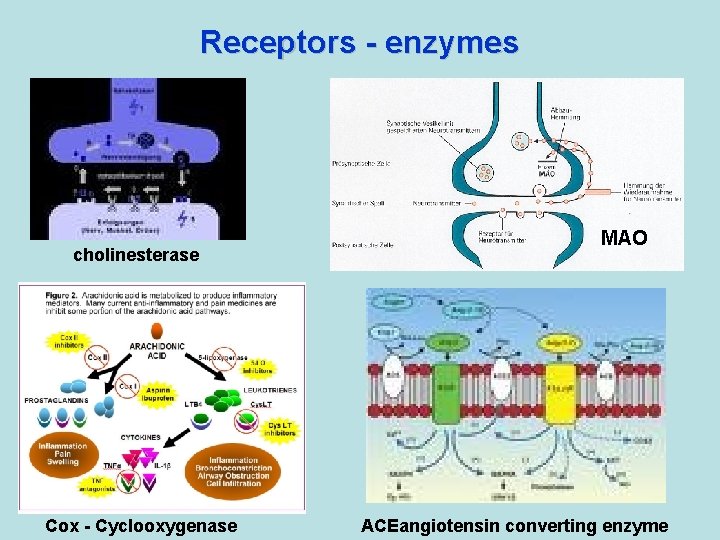
Receptors - enzymes cholinesterase Cox - Cyclooxygenase MAO ACEangiotensin converting enzyme
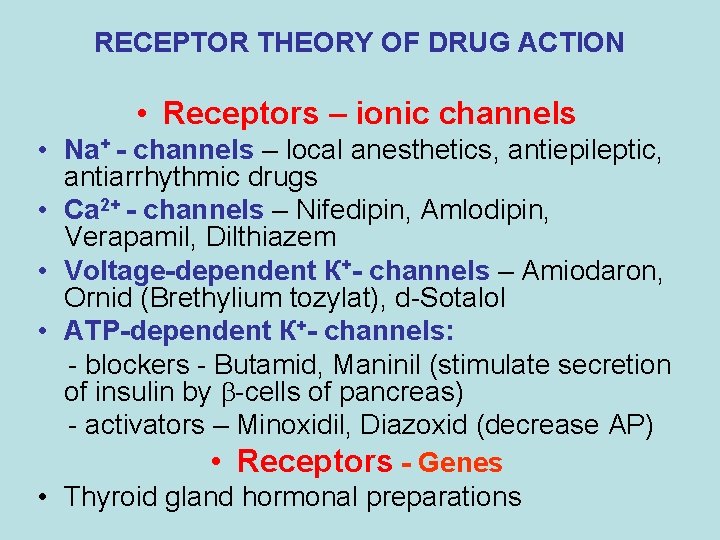
RECEPTOR THEORY OF DRUG ACTION • Receptors – ionic channels • Na+ - channels – local anesthetics, antiepileptic, antiarrhythmic drugs • Са 2+ - channels – Nifedipin, Amlodipin, Verapamil, Dilthiazem • Voltage-dependent К+- channels – Amiodaron, Ornid (Brethylium tozylat), d-Sotalol • АТP-dependent К+- channels: - blockers - Butamid, Maninil (stimulate secretion of insulin by -cells of pancreas) - activators – Minoxidil, Diazoxid (decrease AP) • Receptors - Genes • Thyroid gland hormonal preparations
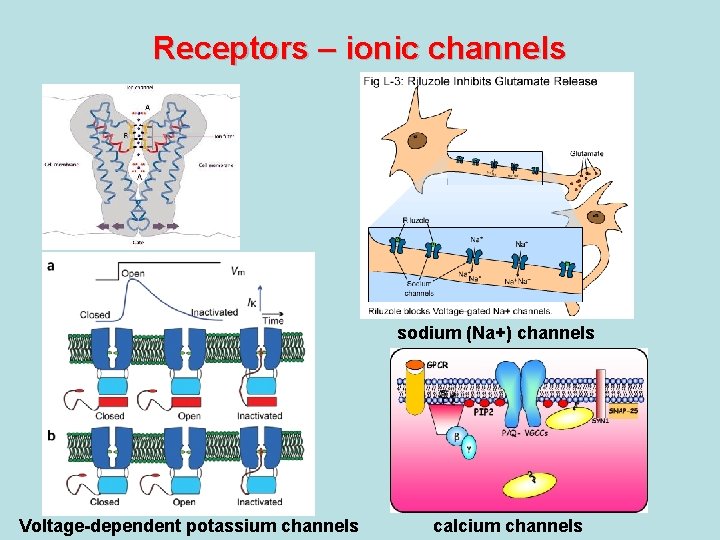
Receptors – ionic channels sodium (Na+) channels Voltage-dependent potassium channels calcium channels
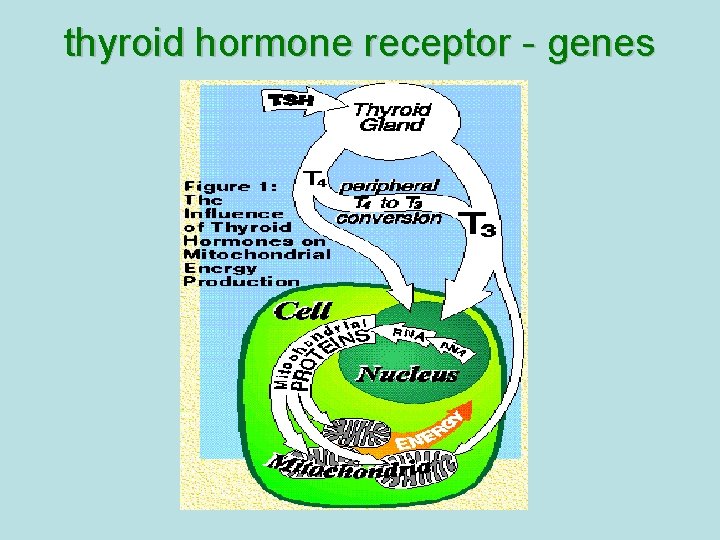
thyroid hormone receptor - genes
NONSPECIFIC ACTION OF DRUGS Due to their physical and chemical properties • Mannit increases osmotic pressure in kidneys canalicules • Direct chemical interaction: Antacides (Mg. O, Na. HCO 3) neutralize HCl, Trilon B (EDTA) binds salts of heavy metals, Na citrate binds Ca-ions • Physical-chemical interaction: Protamine sulfate binds Heparin • Due to the same structure with metabolites of the organism drugs interferences with corresponding metabolic processes : Sulfonamides (have the same structure to PABA), Mercaptopurin (to folic acid and purin)

Factors effecting the properties of drugs Exogenous structure and • chemical physical-chemical properties • pharmaceutical form and the ways of its introduction • doses • regime of feeding • environment factors (meteorological, circadian rhythm) Endogenous • age • sex • pregnancy • diseases
Chemical structure and physical-chemical properties • Degree of ionization: methyl, ethylic group – weak ionization, high lipidotropic activity; hydroxyl, amino-group – high ionization, high hydrophilic properties, weak penetration through the membranes • Fluoric atom in molecules of GCS, neuroleptics increases their activity • Space structure: distance between nitrogen-atoms 0, 6 -0, 8 nm – ganglionblockers, 1, 4 -1, 5 nm – myorelaxants • weak solubility in lipids – bad penetration through the membranes (tubocurarini chloridum), high solubility in lipids – penetration through the HEB (mellictin ) • Large active surface – enterosgel, activated charcoal • Effecting of cell membranes with detergents
Pharmaceutical form and the routs of its administration these factors effecting the bioavailability Bioavailability of drugs complex of pharmacokinetic processes that maintenance active concentration of drug in the area of specific receptors (part of administered drug that reaches the systemic circulation and effects specific receptors)

FOOD - DRUGS
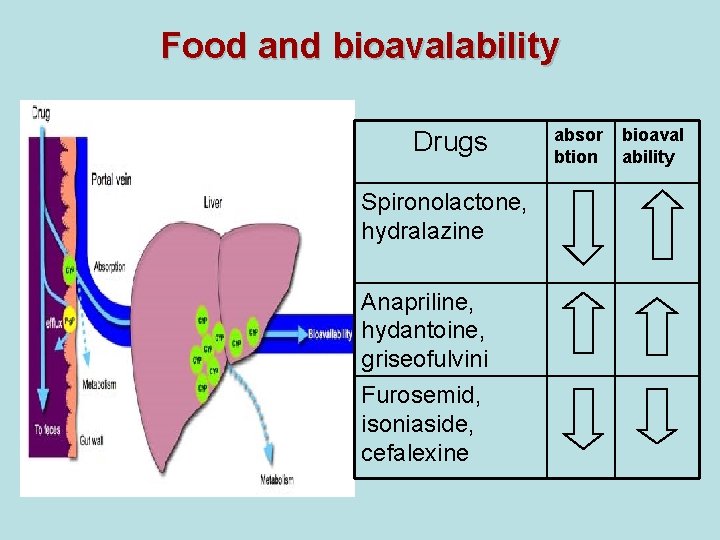
Food and bioavalability Drugs Spironolactone, hydralazine Anapriline, hydantoine, griseofulvini Furosemid, isoniaside, cefalexine absor btion bioaval ability

Drugs and milk • Glucocorticosteroids: • Increase of absorption prednisolone, dexamethasone • NSAID: voltaren, butadion, • Decrease of absorption indometacine • Antibiotics: tetracyclines, • Decrease of absorption fluoroquinolones

Antibiotics and tonic drinks • Macrolides (erythromycine, spiromycine, klaritromycine) • Linkosamides (linkomycine, clindamycine) • Tetracyclines • Decreas of absorption • Decreas of activity

Tannin-containing products • Alkaloids (papaverine, platyphylline, codeine, reserpine) • Decreas of absorption • Decreas of therapeutic activity • Neuroleptics of phenothiazine and buthyrophenone groups (aminazine, haloperidole etc. ) tea

Green tea • Xanthines (Theophyllinum) – Increase of absorption, Increase of adverse reactions (insomnia, nervousness) • Indirect anticoagulants – decrease of effectivenes

Drugs and caffeine-containing products Morphine, papaverine, codeine, atropine, aminazine, haloperidol, hormonal contraceptives, ergotamine Decreas of absorption Decreas of therapeutic activity Ø Paracetamol, aspirine Ø increase of analgesic effect

Grapefruit juice • Calcium antagonist, terfenadine, ciclosporine • Decrease of biotransformation in liver, increase of their blood concentration, increase of toxicity
Diet in case of administration of IMAO It is necessary to exclude such products which contain DOPA and thiramine (which is formed from casein during the process of transforming under the influence of bacteria) Aged cheese, kefir Marinated herring Smoked meat and fish Red vine, beer, yeast Beans, oranges, tangerines, lemons, grape, chocolate, bananas, caviar (red and black)…
INFLUENCE OF BIOLOGICAL RHYTHMS • Glucocorticoids are administered between 6 and 8 in the morning • Theotard (long-acting form of theophylline) is used in the evening (exacerbation of BA at night) • Maximum effect occurs if use diuretics till 10 a. m. • Toxicity of Haloperidolum changes during the day in 5 times • Angina attacks more frequently appears from 2 to 6 p. m.

Influence of sex of the patient on drug action • Morphin, nicotine, coffeine – women are more sensitive • Changes of theophyllin, paracetamol, prednisolon metabolism accordingly different phases of menstrual circle • Pregnancy – worse absorption and slow biotransformation of drugs • During menstruation anticoagulants can course severe uterus bleeding
Influence of pathological processes on drug action • Myocarditis increases the toxicity of cardiac glycosides • Cardiac glycosides act only when cardiac insufficiency • Paracetamolum and other antipyretics act when hyperpyrexia • Pathology of liver leads to inhibition of drugs metabolism and increases their toxicity • Inhibition of drugs elimination is observed in kidney pathology • Smoking provokes risk of thrombosis during administration of hormonal contraceptives
PATHOLOGY OF ENZYMES (enzymopathya) • Decreased activity of glucoso-6 phosphatdehydrogenase: sulfonamides, levomycetinum, drugs for malaria, salicylates, vit C, nitrates lead to hemolysis • Insufficiency of catalase: H 2 O 2 is not metabolized with appearance a foam and can course severe burn of wound • Insufficiency of butyrilcholinesterase: duration of dithylinum action changes to several hours instead of 25 minutes • Insufficiency of acethyltransferase in liver leads to violation of isoniazid, sulfonamides, novocainamid biotransformation
- Slides: 77