COMMON MOLECULAR STRUCTURE Ionic compounds are extremely ordered


- Slides: 27

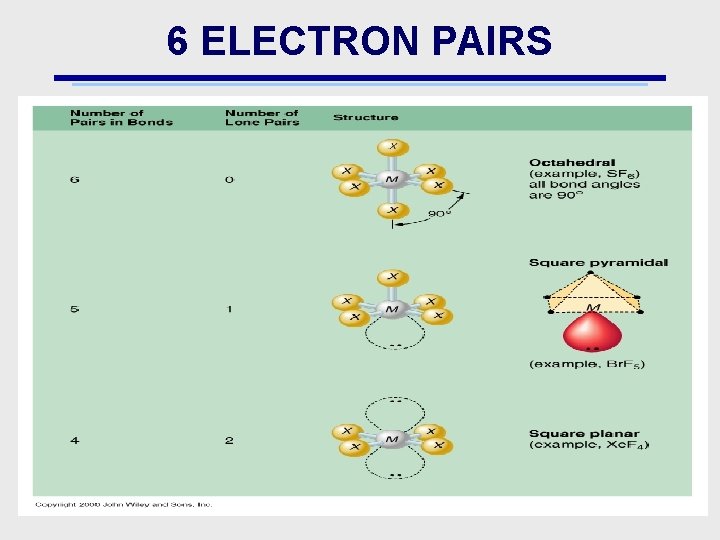
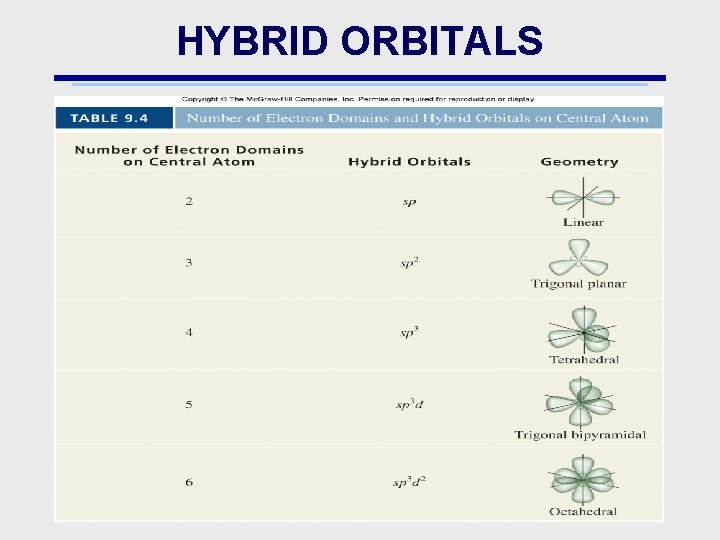
COMMON MOLECULAR STRUCTURE • Ionic compounds are extremely ordered & have easily predictable shapes • Molecular Compounds: maintain their shape when heated • Covalent Compounds: “Bonds” can be actual chemical bonds or lone pairs (both referred to an “electron domains”) – – – 2 bonds: linear 3 bonds: trigonal planar 4 bonds: tetrahedral 5 bonds: trigonal bipyramidal 6 bonds; octahedral

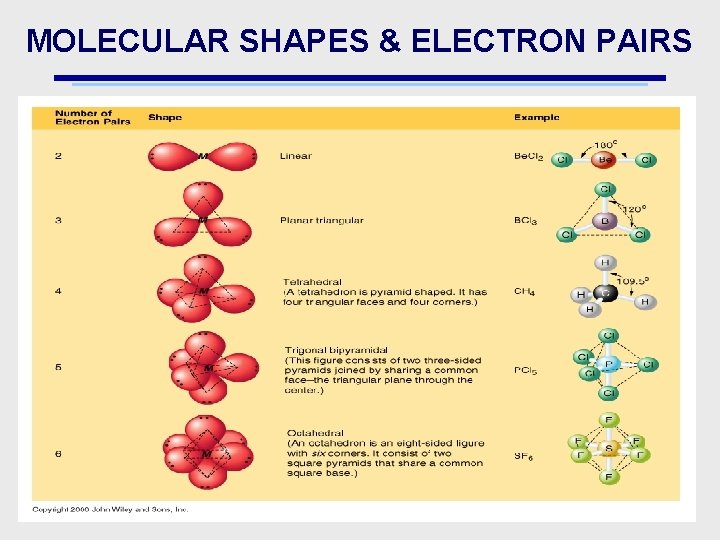
MOLECULAR SHAPES & ELECTRON PAIRS

VALENCE SHELL ELECTRON PAIR REPULSION THEORY (VSEPRT) • Explains effects of lone pair(s) on molecular shape • Bonds, or electron pairs (because of mutual repulsion) try to stay as far apart as possible • Lewis Dot Structures can be used to predict the shapes of molecules • 2 e Pairs: Be. H 2 • 3 e Pairs: BCl 3

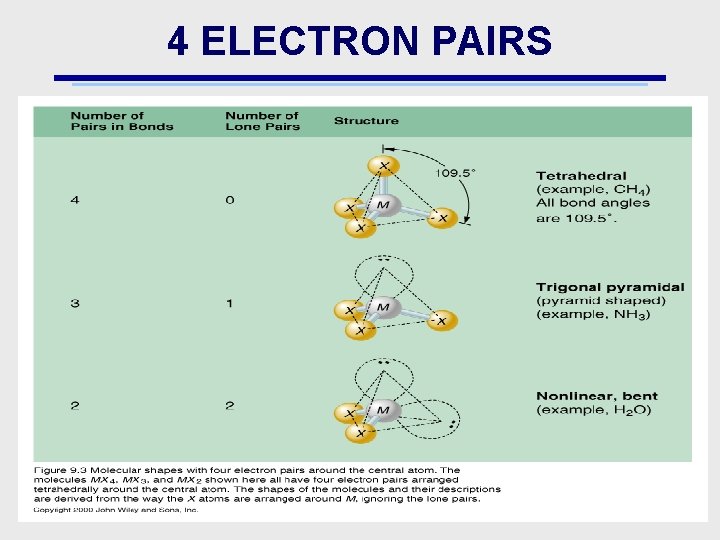
4 ELECTRON PAIRS

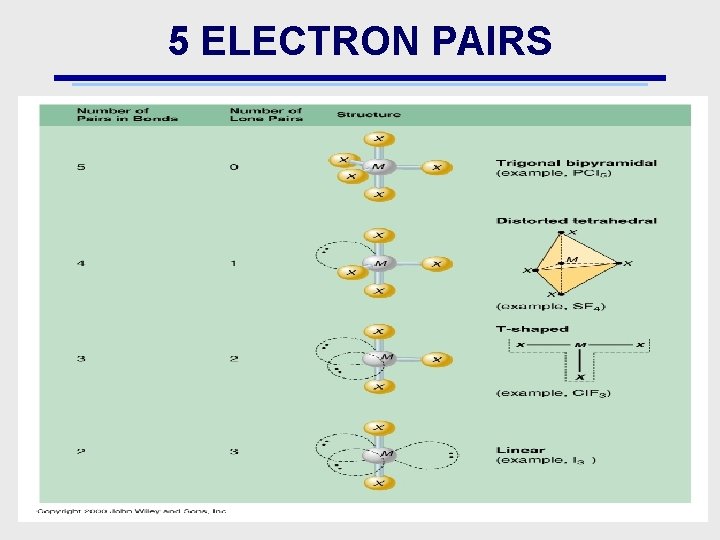
5 ELECTRON PAIRS

6 ELECTRON PAIRS

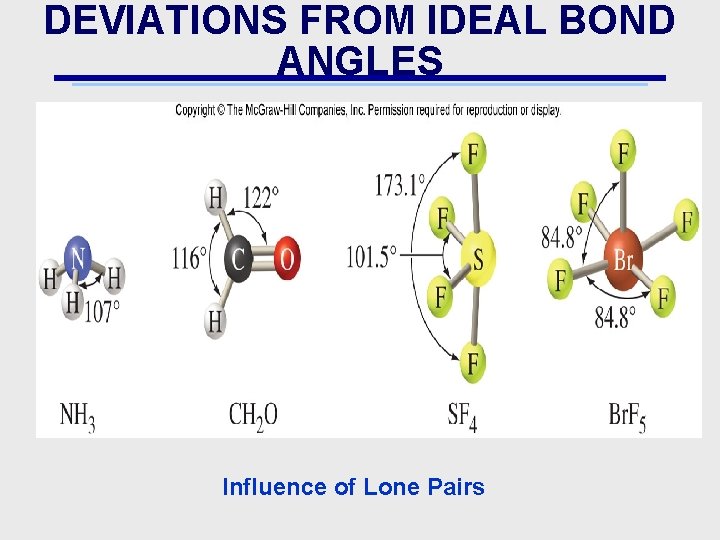
DEVIATIONS FROM IDEAL BOND ANGLES Influence of Lone Pairs

GEOMETRY OF MOLCULES W/MORE THAN 1 CENTRAL ATOM • We treat more complex molecules as though they have multiple central atoms • Consider all bond angles in: – CH 3 -COOH – H 2 SO 4

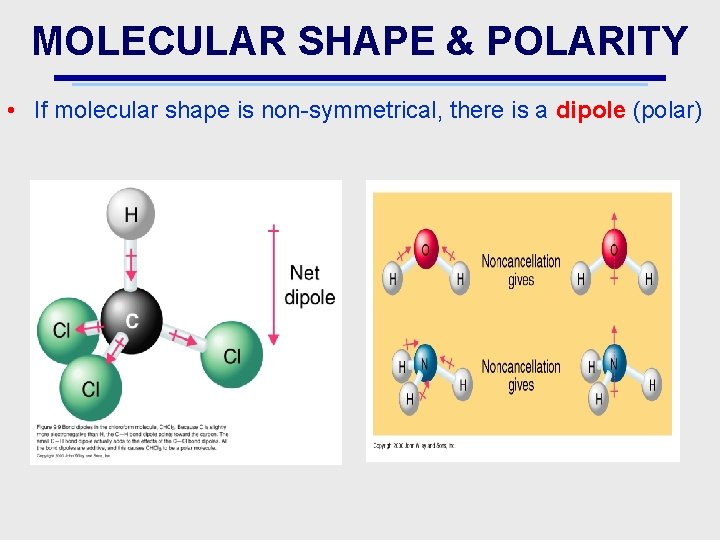
MOLECULAR SHAPE & POLARITY • If molecular shape is non-symmetrical, there is a dipole (polar)

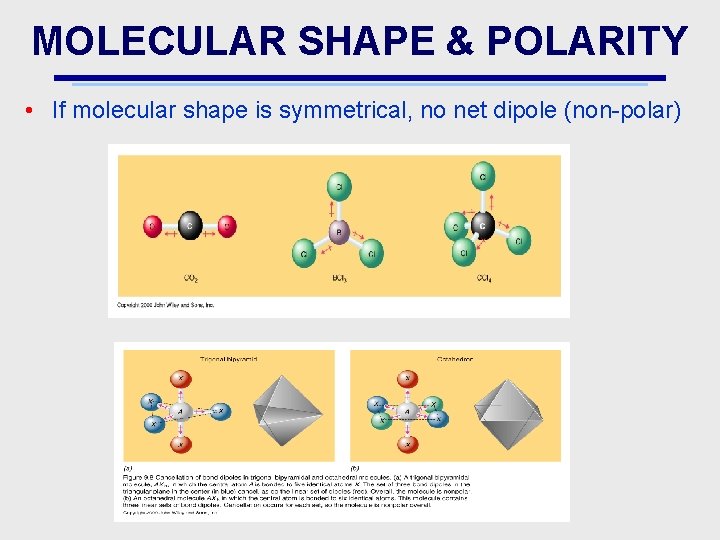
MOLECULAR SHAPE & POLARITY • If molecular shape is symmetrical, no net dipole (non-polar)

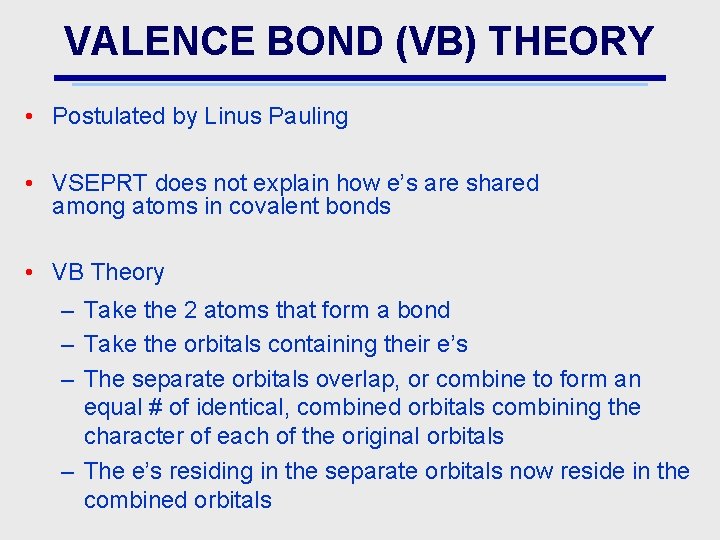
VALENCE BOND (VB) THEORY • Postulated by Linus Pauling • VSEPRT does not explain how e’s are shared among atoms in covalent bonds • VB Theory – Take the 2 atoms that form a bond – Take the orbitals containing their e’s – The separate orbitals overlap, or combine to form an equal # of identical, combined orbitals combining the character of each of the original orbitals – The e’s residing in the separate orbitals now reside in the combined orbitals

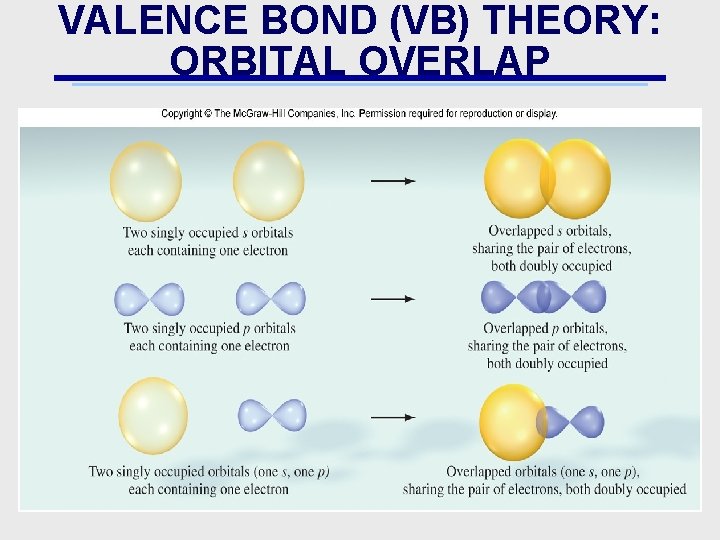
VALENCE BOND (VB) THEORY: ORBITAL OVERLAP

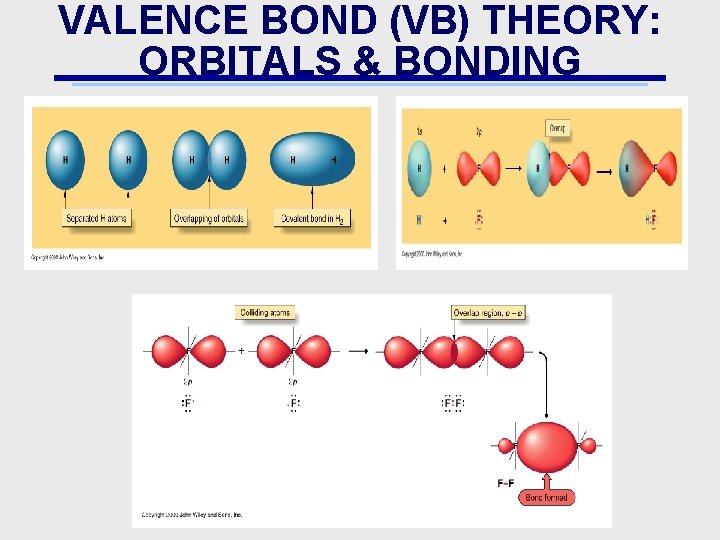
VALENCE BOND (VB) THEORY: ORBITALS & BONDING

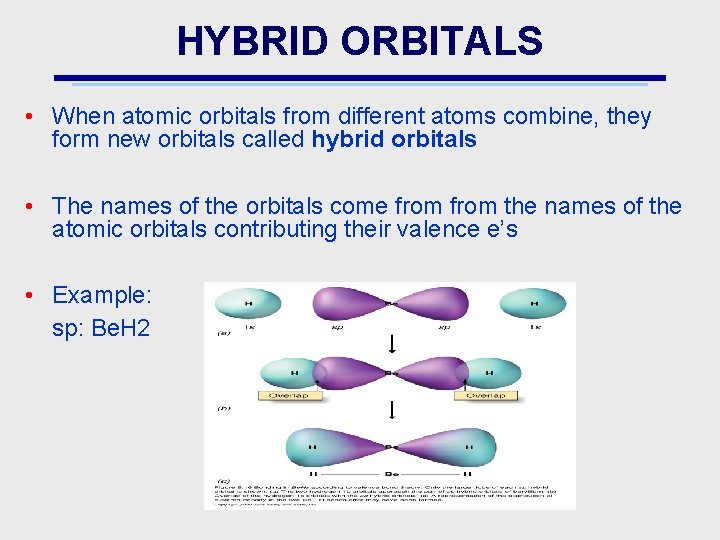
HYBRID ORBITALS • When atomic orbitals from different atoms combine, they form new orbitals called hybrid orbitals • The names of the orbitals come from the names of the atomic orbitals contributing their valence e’s • Example: sp: Be. H 2

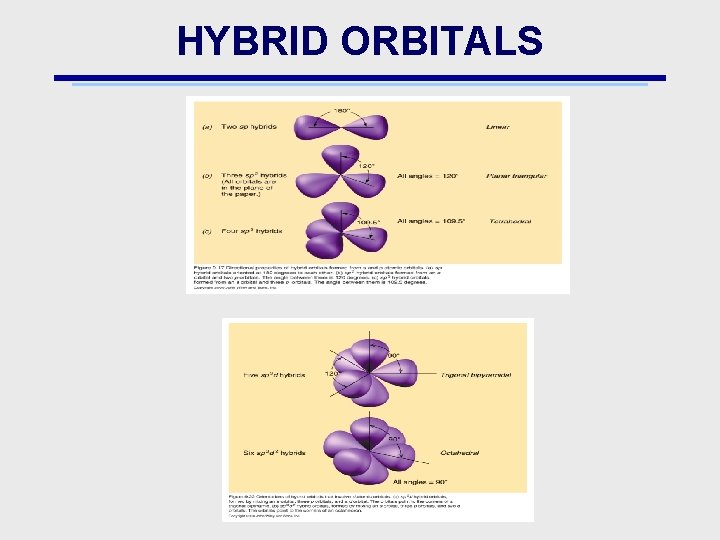
HYBRID ORBITALS

HYBRID ORBITALS

HOW HYBRID ORBITALS FORM • If you need more bonds than there are valence e’s, promote e’s into unfilled d orbitals. • s, p and d orbitals all combine to form an equivalent # of equal hybrid orbitals • 2 bonds - Be. H 2 5 bonds - As. Cl 5 • 3 bonds 6 bonds – BCl 3 • 4 bonds – CH 4 - SF 6 Coordinate Covalent Bond - (BF 4)-

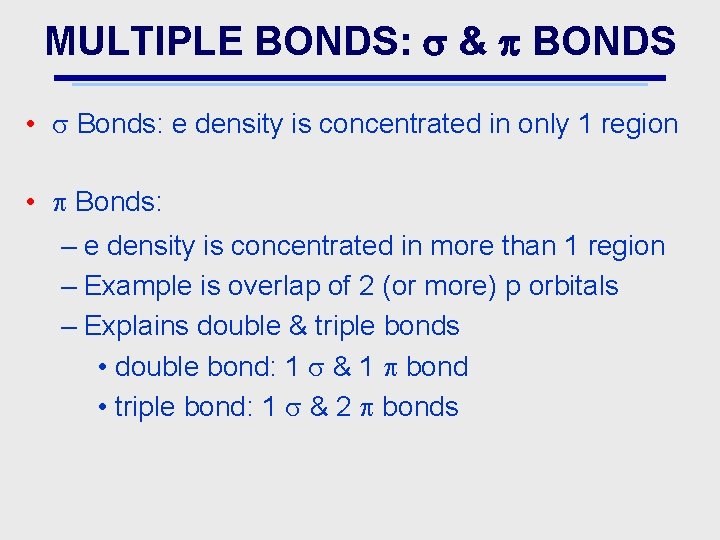
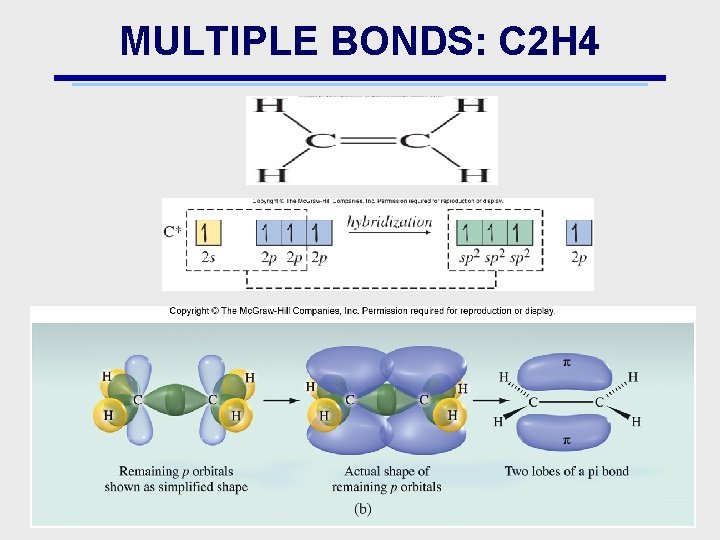
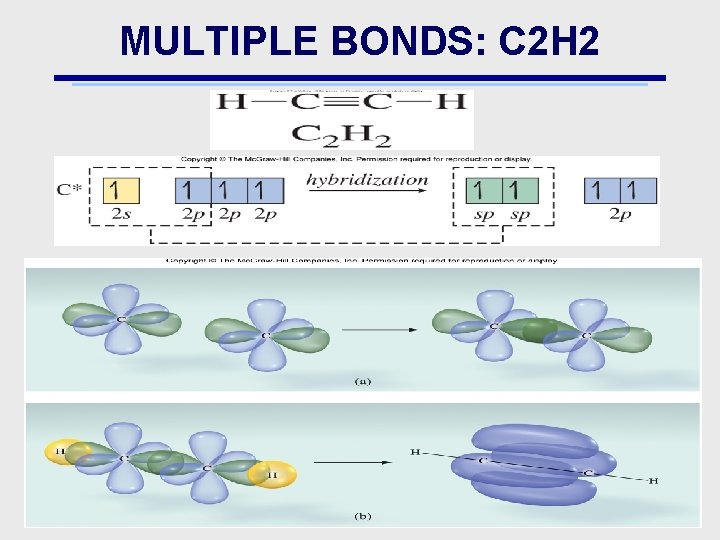
MULTIPLE BONDS: & BONDS • Bonds: e density is concentrated in only 1 region • Bonds: – e density is concentrated in more than 1 region – Example is overlap of 2 (or more) p orbitals – Explains double & triple bonds • double bond: 1 & 1 bond • triple bond: 1 & 2 bonds

MULTIPLE BONDS: C 2 H 4

MULTIPLE BONDS: C 2 H 2

MOLECULAR ORBITAL (MO) THEORY • Individual atomic orbitals combine to form an equivalent # of molecular orbitals (MO’s) • Bonding MO’s: • Antibonding MO’s: • MO’s are populated by valence e’s from contributing atoms

MOLECULAR ORBITAL (MO) THEORY • Bond Order – (# of bonding e’s) - (# of antibonding e’s) / 2 – Need not be an integer • Predicting magnetic properties – # of net unpaired bonding e’s – Paramagnetic: > 1 bonding unpaired e – Diamagnetic: No unpaired bonding e’s

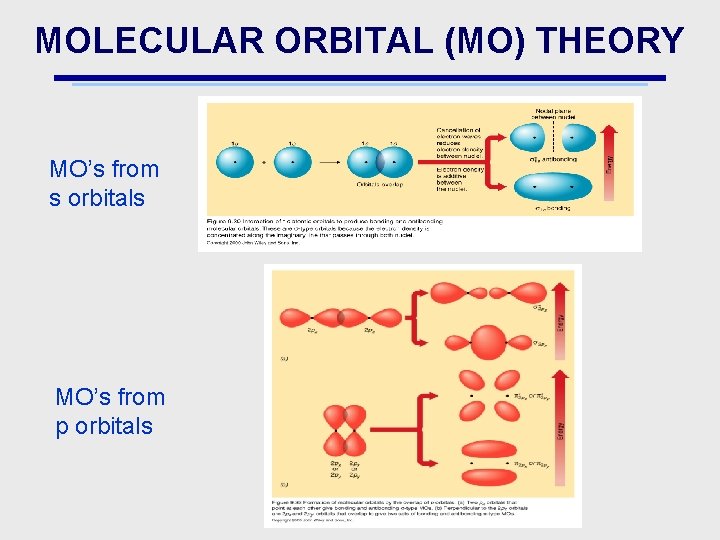
MOLECULAR ORBITAL (MO) THEORY MO’s from s orbitals MO’s from p orbitals

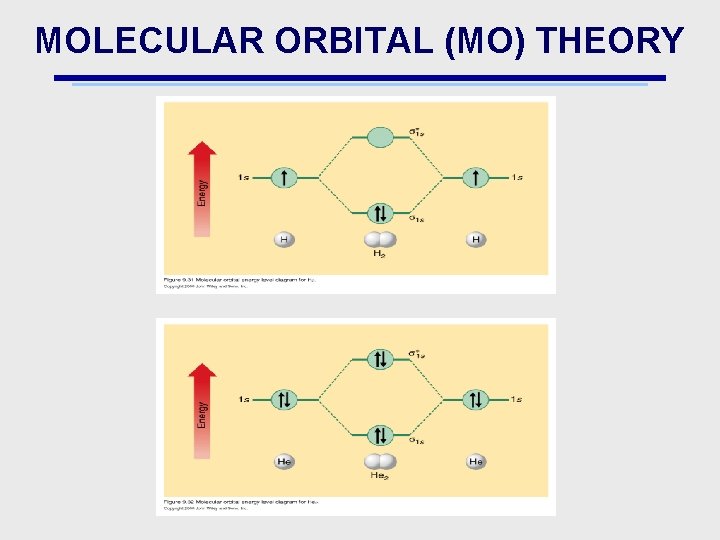
MOLECULAR ORBITAL (MO) THEORY

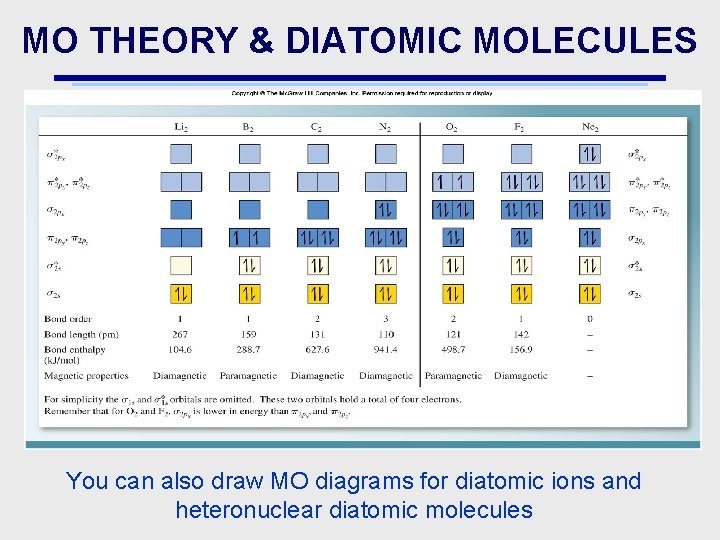
MO THEORY & DIATOMIC MOLECULES You can also draw MO diagrams for diatomic ions and heteronuclear diatomic molecules

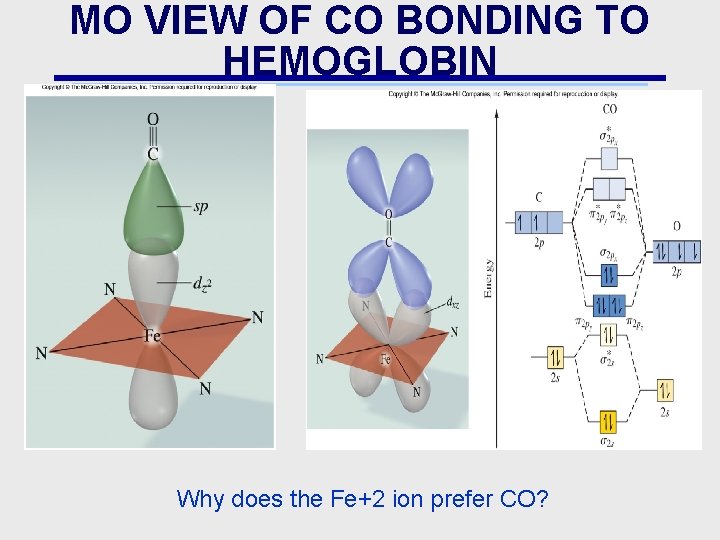
MO VIEW OF CO BONDING TO HEMOGLOBIN Why does the Fe+2 ion prefer CO?

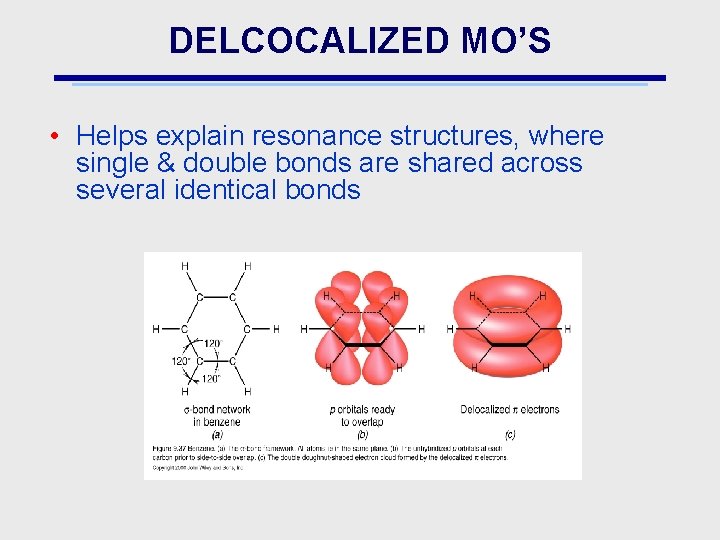
DELCOCALIZED MO’S • Helps explain resonance structures, where single & double bonds are shared across several identical bonds