Common Lab Methods and Calculations Measuring Stuff Measuring

Common Lab Methods and Calculations

Measuring Stuff Measuring Mass: most accurate Measuring Volume: less accurate Why? ? ? (DEMOS)

Measuring Stuff TIPS: Always weigh chemicals if possible (you can also weigh liquids in beakers) Use Volumetric glassware to measure volumes of liquids
Making Solutions of Specific Conc. (It’s all about UNITS) Remember : ‘Dissolve a solute in a solvent to make a solution’ Common Types: Molarity (mol/L), % mass, g/L and ppm (mg/L) g/L = grams solute per Liter solution Molarity (mol/L or M) = moles solute per Liter solution % mass = (mass solute per mass solution) x 100% ppm = milligrams solute per kg solution
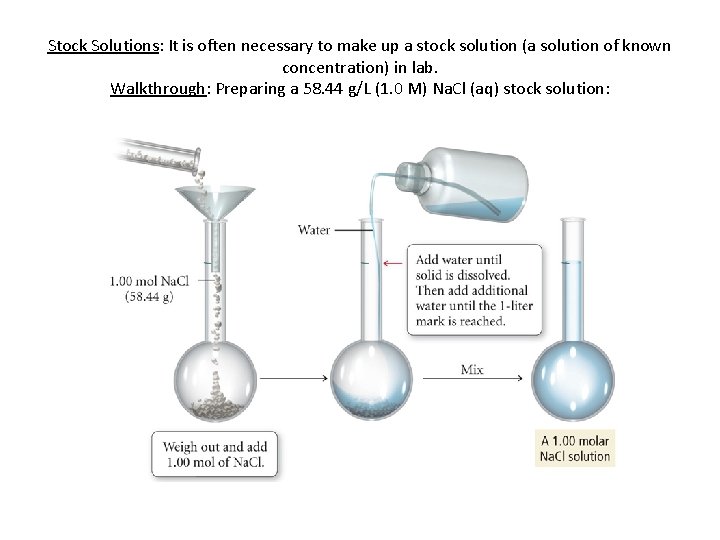
Stock Solutions: It is often necessary to make up a stock solution (a solution of known concentration) in lab. Walkthrough: Preparing a 58. 44 g/L (1. 0 M) Na. Cl (aq) stock solution:

Calculations 1. Molarity: Molarity* (M) = number moles of solute per Liter of solution * The Molarity (mol/L conc. ) is most often printed on lab reagent solution bottles, e. g. 9. 0 M H 2 SO 4 (aq) i. e. Molarity = Moles Solute Liters Solution Units: mol/L or just M Example: What is the concentration (molarity, M) of a solution made by dissolving 25 g of Na. Cl in water and making the final volume of the solution equal to 750 m. L?
More Calculations 2. % Mass = Mass Solute x 100 % Mass Solution (units of %) Example: What is the % mass concentration of an Na. Cl in a solution made by dissolving 25 g of Na. Cl in water and making the final volume of the solution equal to 750 m. L? Assume the final solution has a density of 1. 15 g/m. L How do we determine density (DEMO)?
- Slides: 7