Common Ion Effect Hydrolysis Titration Chapter 19 Common










- Slides: 12

Common Ion Effect, Hydrolysis, Titration Chapter 19

Common Ion Effect n There are times when a chemist may wish to change the concentration of a specific ion in a solution. Such changes are often made by adding a new substance to the solution. n Acetic acid ionizes in a water solution to form acetate and hydronium ions. What will happen if we add some sodium acetate to the solution? n n Sodium acetate dissociates into acetate, CH 3 COO-, and sodium ions. Na. CH 3 COO Na+ + CH 3 COOAcetic acid ionizes into acetate and hydronium ions. H 20 + CH 3 COOH H 3 O+ + CH 3 COO- n Ka is a constant, and does not change unless the temperature changes. If sodium acetate is added to an acetic acid solution, acetate ion concentration is increased and a shift in the equilibrium occurs.

Le Chatelier Revisited: n n Na. CH 3 COO Na+ + CH 3 COOH 20 + CH 3 COOH H 3 O+ + CH 3 COO- n n Because there are more particles of CH 3 COO-, there will be more collisions between CH 3 COO- and H 30+. Thus, the rate of reaction will increase toward acetic acid. Some of the excess acetate ions unite with hydronium ions to form molecular acetic acid and water. This reaction results in the removal of hydronium ions from solution (thus decreasing hydronium ion concentration). The acetic acid concentration increases slightly. A new equilibrium is established with more acetate ions and fewer hydronium ions. Ka remains unchanged. n n n The acetate ion is common to both acetic acid and sodium acetate. The effect of the acetate ion on the acetic acid and solution is called the common ion effect. The addition of a common increases the concentration of one of the products of the ionization. Thus, the equilibrium shifts toward the opposite side in accordance with Le Chatelier's principle. By adding acetate ions (sodium acetate), we have placed a stress on the system (a surplus of acetate ions). The system shifts so as to relieve that stress by reacting hydronium ions with the acetate ions. In the process acetate and hydronium ions are consumed and acetic acid molecules are produced.

Acid-Base Summary n n n n n 1. There are three common acid-base theories: the Arrhenius theory, the Bronsted-Lowry theory, and the Lewis theory. 2. Metallic oxides tend to form basic anhydrides, while nonmetallic oxides tend to form acidic anhydrides. 3. A Salt is a crystalline compound composed of the negative ion of an acid and the positive ion of a base. 4. A Strong acid ionizes completely in a water solution. A weak acid ionizes only slightly in a water solution. 5. In net ionic equations, only the reacting species are shown. Spectator ions do not appear. 8. The ionization of a weak acid or base is an equilibrium process. 7. Percent ionization is the amount ionized divided by the original amount multiplied by 100%. 8. A common ion represses the ionization of a weak electrolyte. 9. Polyprotic acids contain more than one ionizable hydrogen atom. Each successive ionization occurs to a lesser extent.

Hydrolysis n Recall that hydrolysis occurs when water dissociates into its respective ions. – i. e. , H 2 O OH- + H+ Hydrolysis means "cleavage by means of water". There are many different types of hydrolysis reaction, but in this problem, hydrolysis refers to a reaction between water and the anion of the weak acid. n For example, the acetate ion hydrolyzes according to: n – CH 3 COO-(aq) + H 2 O(l) = CH 3 COOH (aq) + OH-(aq) – You would expect a solution of sodium acetate to be somewhat basic because of the hydroxide produced by this reaction. But how basic will the solution be? – Recall that Kw=Ka * Kb n Try predicting which of the following solutions will have the highest and which will have the lowest p. H: – – 0. 1 M Na. Cl, 0. 1 M Na. CH 3 COO, 0. 1 M Na. NO 2, given that HCl is a strong acid, HNO 2 is a weak acid, and CH 3 COOH(aq) is a weaker acid than HNO 2.

Answer n n The Na. CH 3 COO solution has the highest p. H, and the Na. Cl has the lowest. 0. 1 M Na. Cl solution. The hydrolysis reaction would form HCl is a strong acid (the Ka is extremely large), so you would expect that the chloride ion would not hydrolyze at all! The p. H of the solution would be close to 7. 0. 1 M Na. CH 3 COO solution. The hydrolysis reaction would form CH 3 COOH is a weak acid (the Ka is about 1. 8 x 10 -5), so CH 3 COO- would undergo hydrolysis and the p. H would be greater than 7. 0. 1 M Na. NO 2 solution. The hydrolysis reaction would form HNO 2 is a weak acid (the Ka is about 7. 2 x 10 -4), so NO 2 - would undergo hydrolysis and the p. H would be greater than 7. Since the Ka is greater than the Ka for CH 3 COOH, this solution would be more weakly basic than the Na. CH 3 COO solution.

Titration Overview Titration is a procedure for determining the amount of some unknown substance (the analyte) by quantitative reaction with a measured volume of a solution of precisely known concentration (the titrant). n The point at which the analyte equals the titrant is known as the equivalence point. n Indicators are often used to visually signal the approach and achievement of the equivalence point in a titration reaction. n

The Titration Experiment We use this instrumentation setup to calculate the amount of unknown acid in the receiving flask by measuring the amount of base, or titrant, it takes to neutralize the acid. n There are two major ways to know when the solution has been neutralized. n – The first uses a p. H meter in the receiving flask adding base slowly until the p. H reads exactly 7. – The second method uses an indicator. § The color changes when the solution contains a 1: 1 mixture of the differently colored forms of the indicator. § The p. H equals the p. Ka of the indicator at the endpoint of the indicator. n Since we know the p. H of the solution and the volume of titrant added, we can then deduce how much base was needed to neutralize the unknown sample.

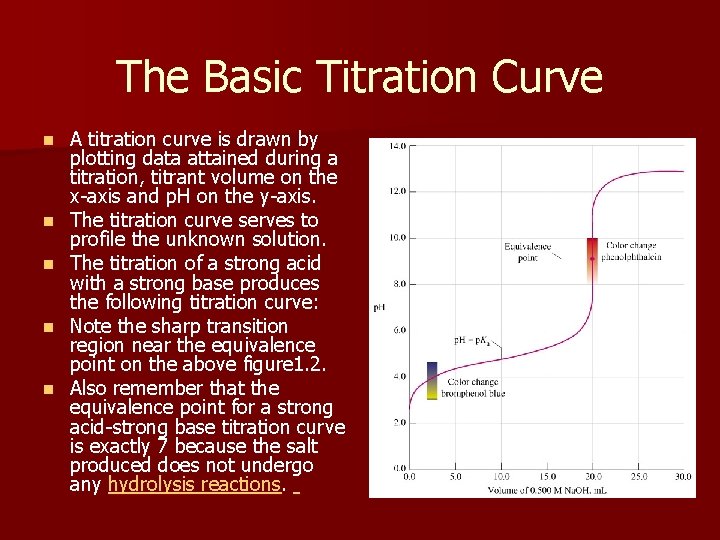
The Basic Titration Curve n n n A titration curve is drawn by plotting data attained during a titration, titrant volume on the x-axis and p. H on the y-axis. The titration curve serves to profile the unknown solution. The titration of a strong acid with a strong base produces the following titration curve: Note the sharp transition region near the equivalence point on the above figure 1. 2. Also remember that the equivalence point for a strong acid-strong base titration curve is exactly 7 because the salt produced does not undergo any hydrolysis reactions.

Titrating Weak Acids with Strong Bases If a strong base is used to titrate a weak acid, the p. H at the equivalence point will not be 7. n There is a lag in reaching the equivalence point, as some of the weak acid is converted to its conjugate base. n – Aka, this is the action of a buffer. – The resultant lag that precedes the equivalence point is called the buffering region. In the buffering region, it takes a large amount of Na. OH to produce a small change in the p. H of the receiving solution. n Because the conjugate base is basic, the p. H will be greater than 7 at the equivalence point. – We will leave the calculations to APChem, but know (If you care) that it involves the Henderson. Hasselbalch equation, the p. Kb and concentration of the conjugate base of the weak acid.

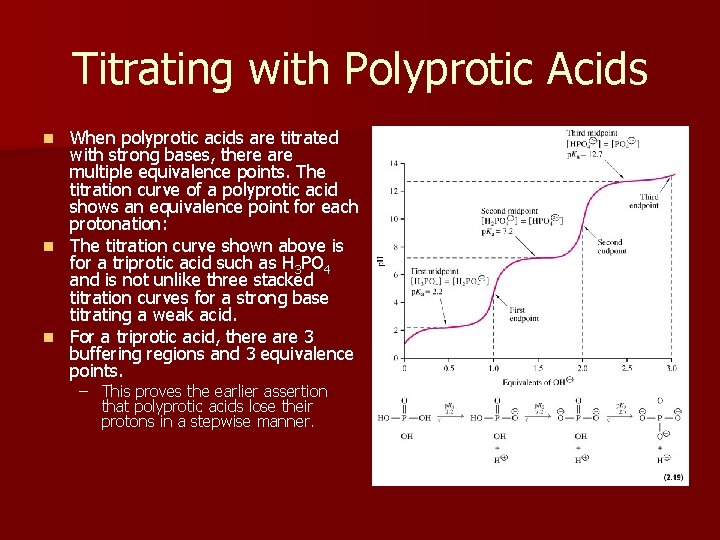
Titrating with Polyprotic Acids When polyprotic acids are titrated with strong bases, there are multiple equivalence points. The titration curve of a polyprotic acid shows an equivalence point for each protonation: n The titration curve shown above is for a triprotic acid such as H 3 PO 4 and is not unlike three stacked titration curves for a strong base titrating a weak acid. n For a triprotic acid, there are 3 buffering regions and 3 equivalence points. n – This proves the earlier assertion that polyprotic acids lose their protons in a stepwise manner.

More Titration n The titration of a base with an acid produces a flipped-over version of the titration curve of an acid with a base. – p. H is therefore decreased upon addition of the acid. The p. H of a solution at the equivalence point has nothing to do with the volume of titrant necessary to reach the equivalence point n It is a property inherent to the composition of the solution. n – i. e. , whether the analyte is strong/weak and concentrated/dilute affect the volume of titrant necessary to completely neutralize the solution