Common Crystal Structure Types NonSilicates Dr Stephen Crabtree
Common Crystal Structure Types: Non-Silicates Dr. Stephen Crabtree September 11, 2019

Crystal Structure Effects • Crystal structure affects: – Cleavage – Hardness – Density – Melting Point – Refractive Index – X-Ray Diffraction Pattern – Solid Solution – etc. . .
Crystal Structure Effects • We derive the sizes of atoms and ions from a crystal structure properties • Determine structure from: – External symmetry – X-ray diffraction effects – Electron diffraction patterns – Density – Chemical composition
Defining the “Unit Cell” • Geometric arrangement of all the atoms, and the bonding and coordination between them • A unit cell is the smallest possible molecularly bonded structure of the crystal – Can be repeated/tiled over and over to form the whole crystal
Example: Halite • External symmetry indicates an isometric cubic structure • X-ray diffraction indicates a unit cell edge dimension of 5. 64 Å (5. 64 * 10 -7 mm) • Chemical analysis – 39. 4 wt% Na; 60. 6 wt% Cl – Divide by known atomic masses – Na : Cl = 1 : 1

Example: Halite • Density known: 2. 165 g/cm 3 • Unit cell volume: 179. 4 Å3 – Volume = 1. 794 * 10 -22 cm 3 • Calculate number of formula units per unit cell – Dens. = (Z * M. W. )/(Av. # * Vol) – Z = (D * Av # * Vol)/M. W. – Z = 4 Na. Cl units = 4 Na & 4 Cl

Example: Halite +z +y +x
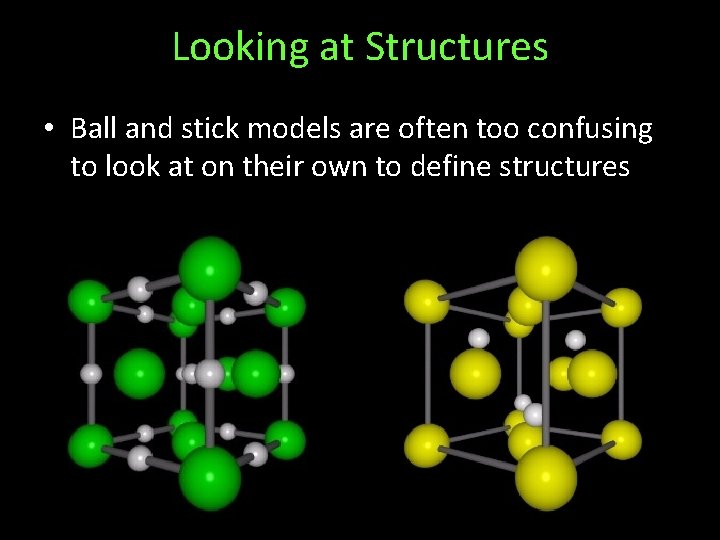
Looking at Structures • Ball and stick models are often too confusing to look at on their own to define structures
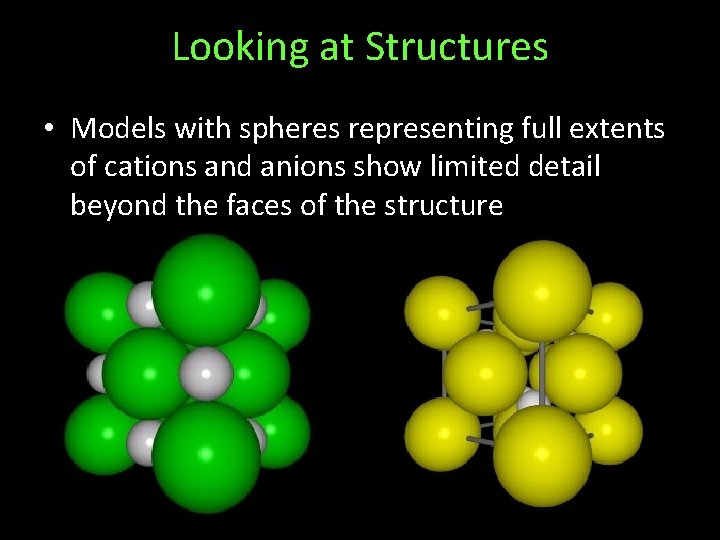
Looking at Structures • Models with spheres representing full extents of cations and anions show limited detail beyond the faces of the structure
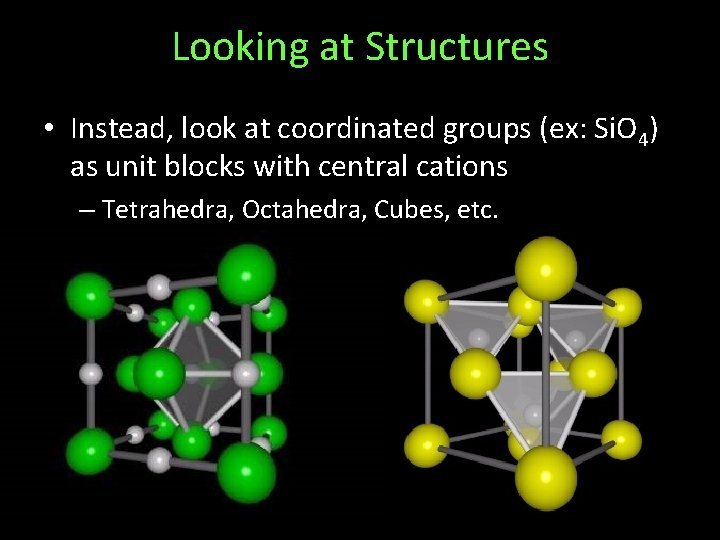
Looking at Structures • Instead, look at coordinated groups (ex: Si. O 4) as unit blocks with central cations – Tetrahedra, Octahedra, Cubes, etc.
Isostructuralism • Two crystals or compounds with the same general structure – Does not imply identical properties – hardness, color, melting point, etc – Ex: UO 2 and Ca. F 2 • Not all compounds with same ratio of atoms and charges are isostructural – size effects, etc. – Ex: Ti. O 2 vs. UO 2 – Na. Cl vs. Zn. S
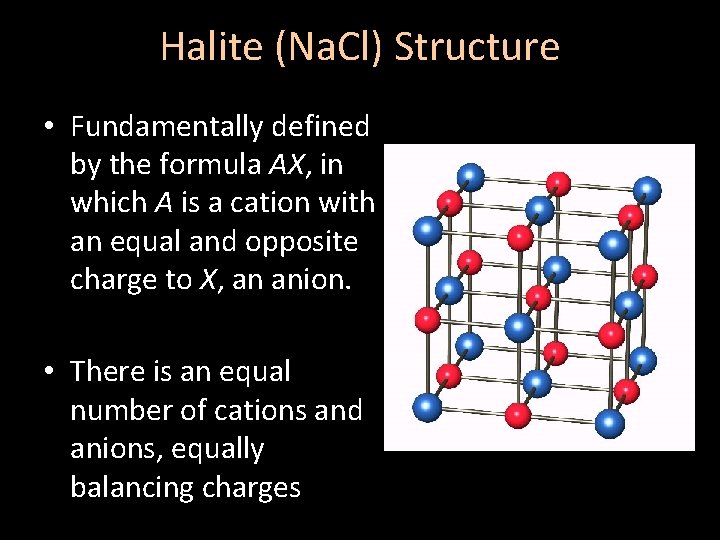
Halite (Na. Cl) Structure • Fundamentally defined by the formula AX, in which A is a cation with an equal and opposite charge to X, an anion. • There is an equal number of cations and anions, equally balancing charges
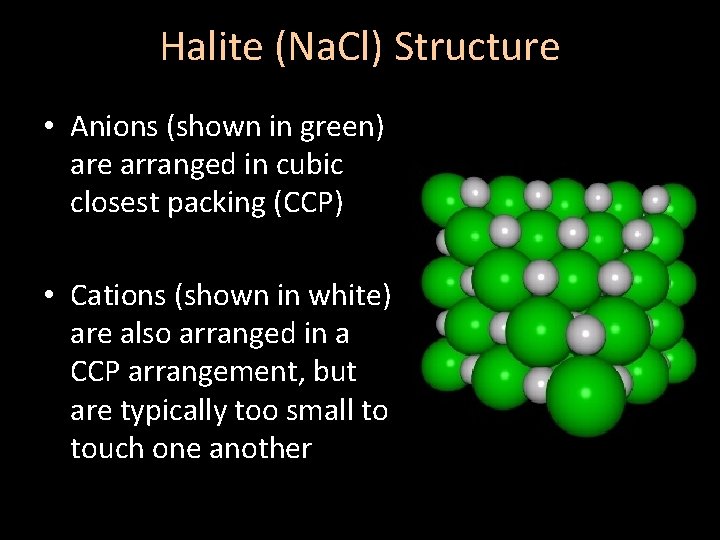
Halite (Na. Cl) Structure • Anions (shown in green) are arranged in cubic closest packing (CCP) • Cations (shown in white) are also arranged in a CCP arrangement, but are typically too small to touch one another
![Halite (Na. Cl) Structure • Cations and anions occupy octahedral sites [CN = 6] Halite (Na. Cl) Structure • Cations and anions occupy octahedral sites [CN = 6]](http://slidetodoc.com/presentation_image_h2/016635b3ad13ab714f6a81e8291a1565/image-14.jpg)
Halite (Na. Cl) Structure • Cations and anions occupy octahedral sites [CN = 6] – RA : RX = 0. 41 – 0. 73
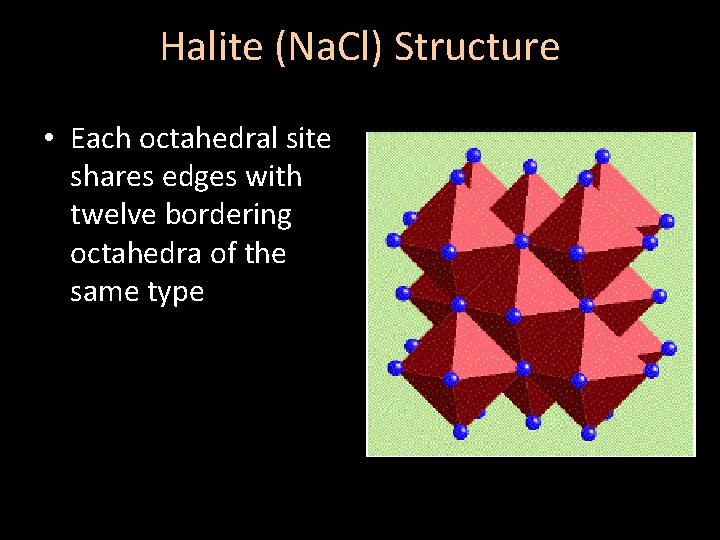
Halite (Na. Cl) Structure • Each octahedral site shares edges with twelve bordering octahedra of the same type

Halite (Na. Cl) Structure • Examples: – Halide Minerals: • • Li. F, Li. Cl, Li. Br, Li. I Na. F, Na. Cl, Na. Br, Na. I KF, KCl, KBr, KI Rb. F, Rb. Cl, Rb. Br, Rb. I – Oxide Minerals Sylvite - KCl Periclase - Mg. O • Mg. O, Ca. O, Sr. O, Ba. O, Ni. O – Sulfides • Mg. S, Ca. S, Mn. S, Pb. S Galena - Pb. S
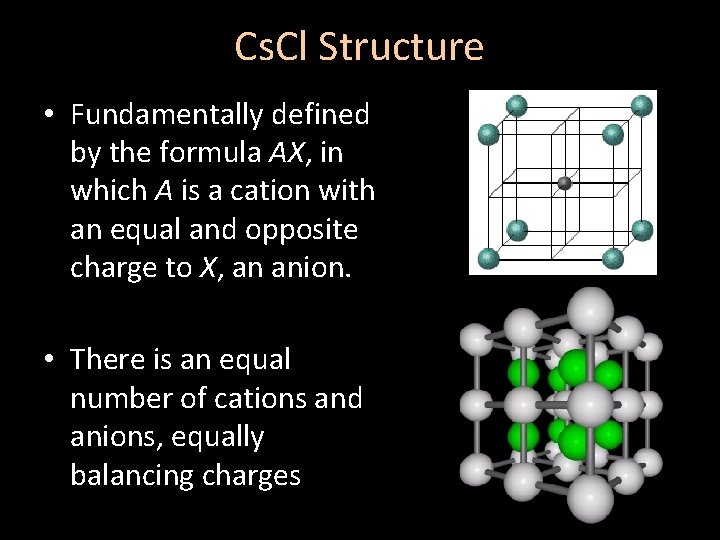
Cs. Cl Structure • Fundamentally defined by the formula AX, in which A is a cation with an equal and opposite charge to X, an anion. • There is an equal number of cations and anions, equally balancing charges
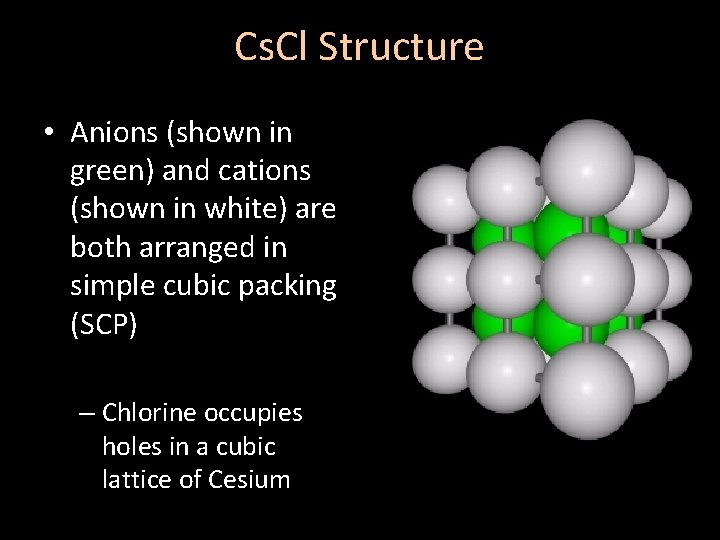
Cs. Cl Structure • Anions (shown in green) and cations (shown in white) are both arranged in simple cubic packing (SCP) – Chlorine occupies holes in a cubic lattice of Cesium
![Cs. Cl Structure • Cations and anions occupy cubic sites [CN = 8] – Cs. Cl Structure • Cations and anions occupy cubic sites [CN = 8] –](http://slidetodoc.com/presentation_image_h2/016635b3ad13ab714f6a81e8291a1565/image-19.jpg)
Cs. Cl Structure • Cations and anions occupy cubic sites [CN = 8] – RA : RX = 0. 73 – 1
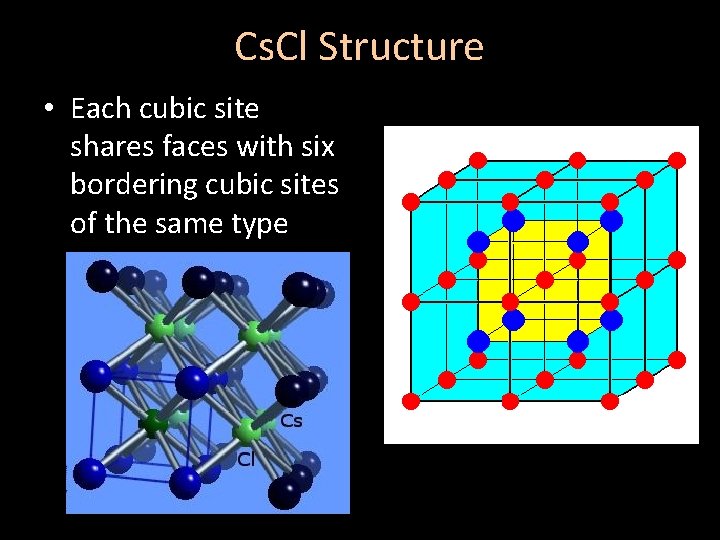
Cs. Cl Structure • Each cubic site shares faces with six bordering cubic sites of the same type

Cs. Cl Structure • Examples: – Not Commonly Found in Nature – Halide Compounds: • Cs. Cl, Cs. Br, Cs. I – Ammonium Halide Compounds • (NH 4)Cl, (NH 4)Br Salammoniac – NH 4 Cl
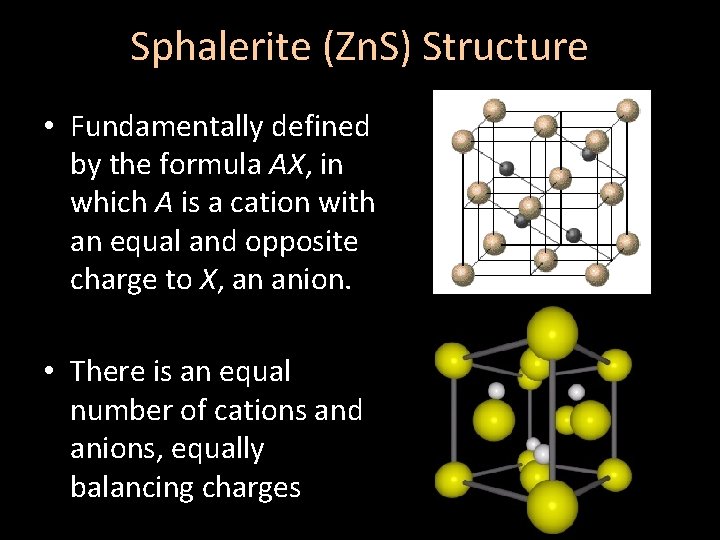
Sphalerite (Zn. S) Structure • Fundamentally defined by the formula AX, in which A is a cation with an equal and opposite charge to X, an anion. • There is an equal number of cations and anions, equally balancing charges
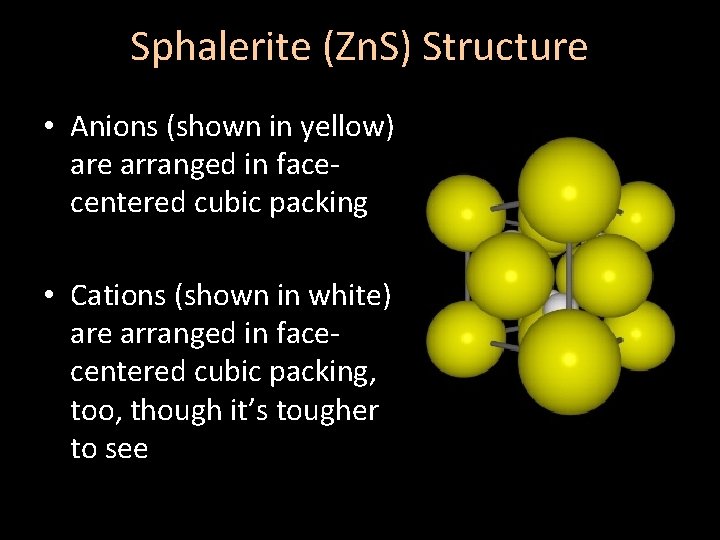
Sphalerite (Zn. S) Structure • Anions (shown in yellow) are arranged in facecentered cubic packing • Cations (shown in white) are arranged in facecentered cubic packing, too, though it’s tougher to see
![Sphalerite (Zn. S) Structure • Cations and anions occupy tetrahedral sites [CN = 4] Sphalerite (Zn. S) Structure • Cations and anions occupy tetrahedral sites [CN = 4]](http://slidetodoc.com/presentation_image_h2/016635b3ad13ab714f6a81e8291a1565/image-24.jpg)
Sphalerite (Zn. S) Structure • Cations and anions occupy tetrahedral sites [CN = 4] – RA : RX = 0. 32
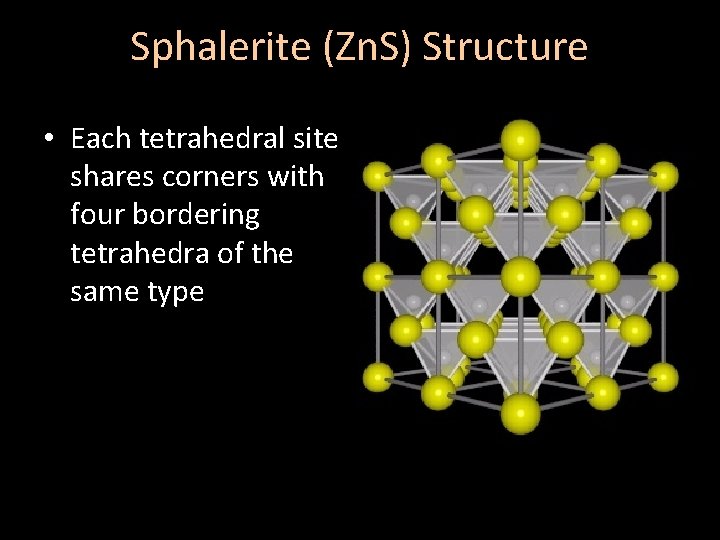
Sphalerite (Zn. S) Structure • Each tetrahedral site shares corners with four bordering tetrahedra of the same type
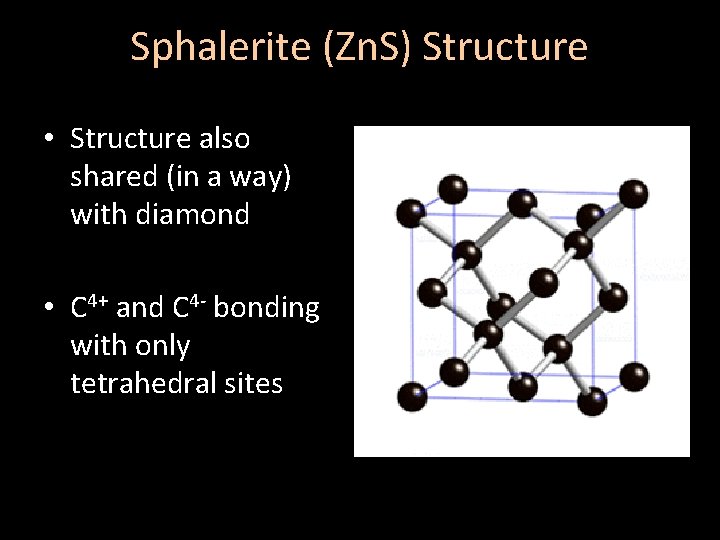
Sphalerite (Zn. S) Structure • Structure also shared (in a way) with diamond • C 4+ and C 4 - bonding with only tetrahedral sites

Sphalerite (Zn. S) Structure • Examples: – Sulfide Minerals: • Zn. S, Cu. Fe. S 2 – Silicon Carbide (Si. C) Sphalerite - Zn. S – Diamond Chalcopyrite – Cu. Fe. S 2 Diamond - C
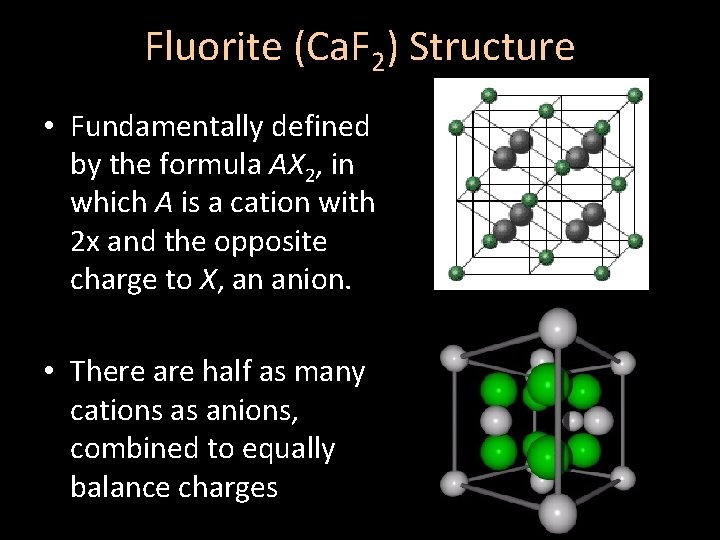
Fluorite (Ca. F 2) Structure • Fundamentally defined by the formula AX 2, in which A is a cation with 2 x and the opposite charge to X, an anion. • There are half as many cations as anions, combined to equally balance charges
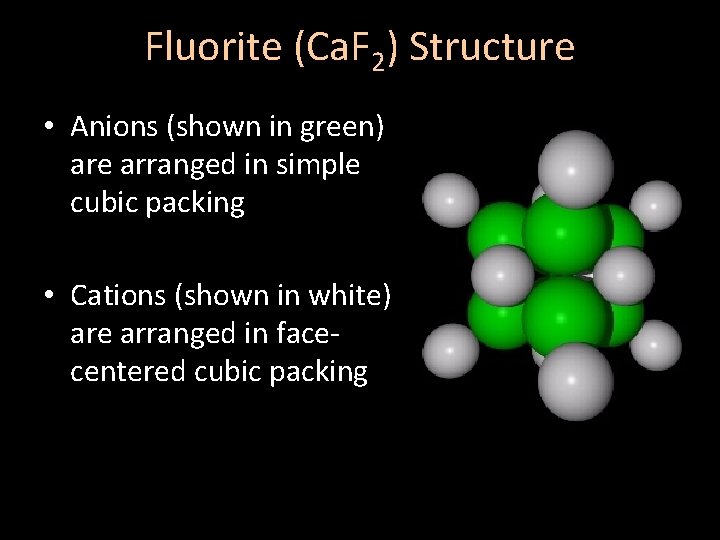
Fluorite (Ca. F 2) Structure • Anions (shown in green) are arranged in simple cubic packing • Cations (shown in white) are arranged in facecentered cubic packing
![Fluorite (Ca. F 2) Structure • Cations occupy cubic sites [CN = 8] – Fluorite (Ca. F 2) Structure • Cations occupy cubic sites [CN = 8] –](http://slidetodoc.com/presentation_image_h2/016635b3ad13ab714f6a81e8291a1565/image-30.jpg)
Fluorite (Ca. F 2) Structure • Cations occupy cubic sites [CN = 8] – RCa : RF ≈ 0. 75 • Anions occupy tetrahedral sites [CN = 4] – RCa : RF ≈ 0. 75
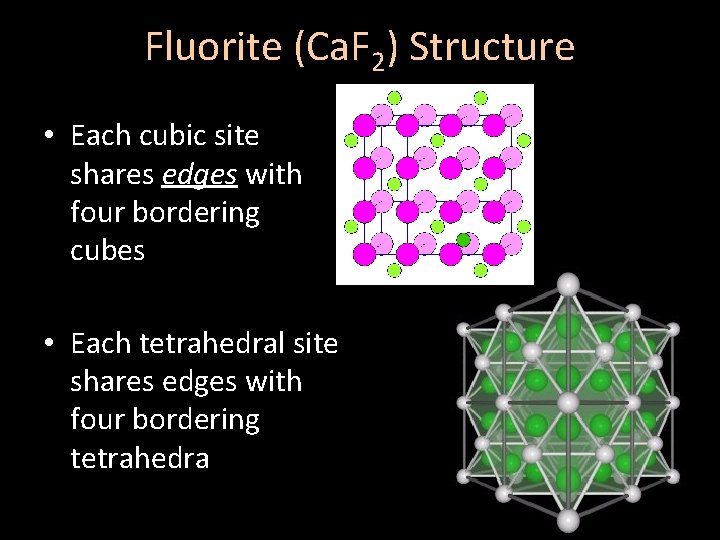
Fluorite (Ca. F 2) Structure • Each cubic site shares edges with four bordering cubes • Each tetrahedral site shares edges with four bordering tetrahedra
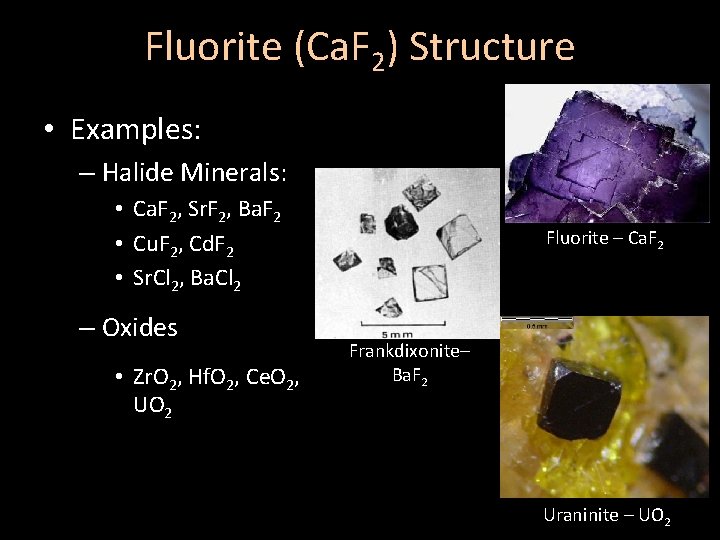
Fluorite (Ca. F 2) Structure • Examples: – Halide Minerals: • Ca. F 2, Sr. F 2, Ba. F 2 • Cu. F 2, Cd. F 2 • Sr. Cl 2, Ba. Cl 2 – Oxides • Zr. O 2, Hf. O 2, Ce. O 2, UO 2 Fluorite – Ca. F 2 Frankdixonite– Ba. F 2 Uraninite – UO 2
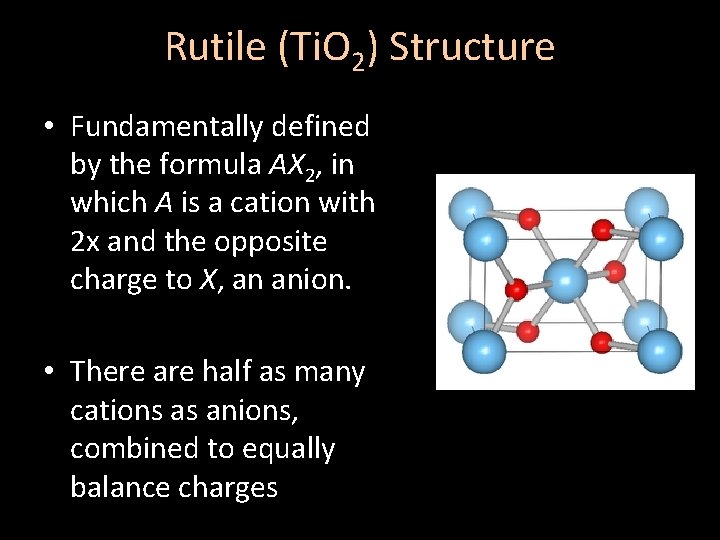
Rutile (Ti. O 2) Structure • Fundamentally defined by the formula AX 2, in which A is a cation with 2 x and the opposite charge to X, an anion. • There are half as many cations as anions, combined to equally balance charges
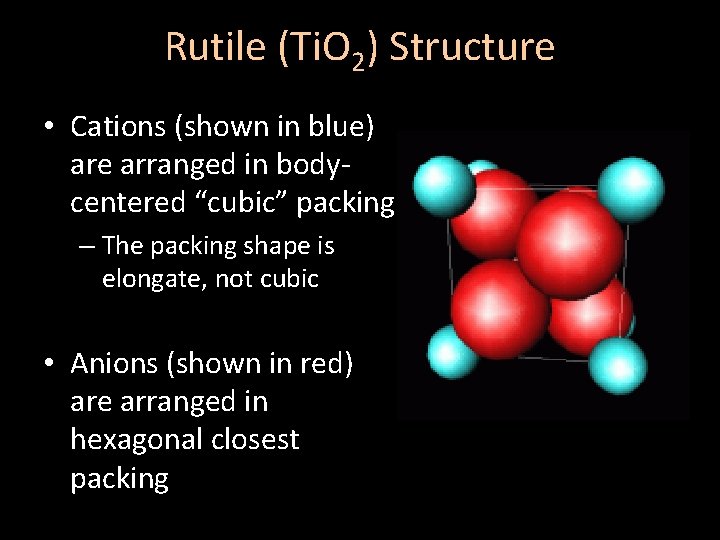
Rutile (Ti. O 2) Structure • Cations (shown in blue) are arranged in bodycentered “cubic” packing – The packing shape is elongate, not cubic • Anions (shown in red) are arranged in hexagonal closest packing
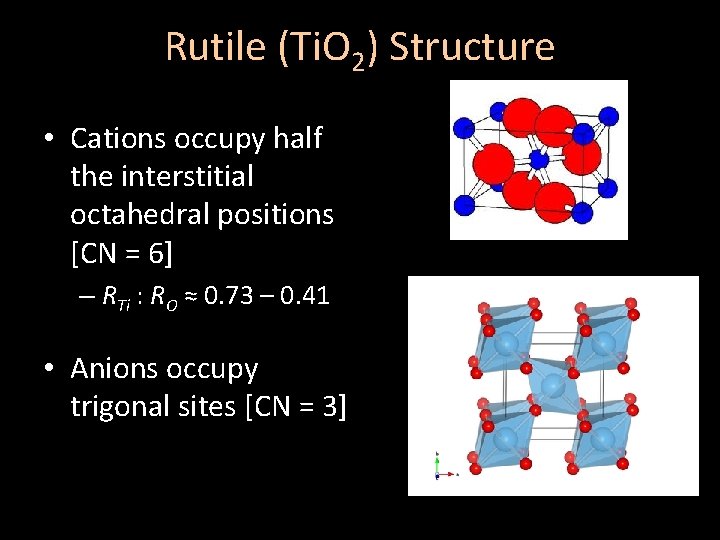
Rutile (Ti. O 2) Structure • Cations occupy half the interstitial octahedral positions [CN = 6] – RTi : RO ≈ 0. 73 – 0. 41 • Anions occupy trigonal sites [CN = 3]
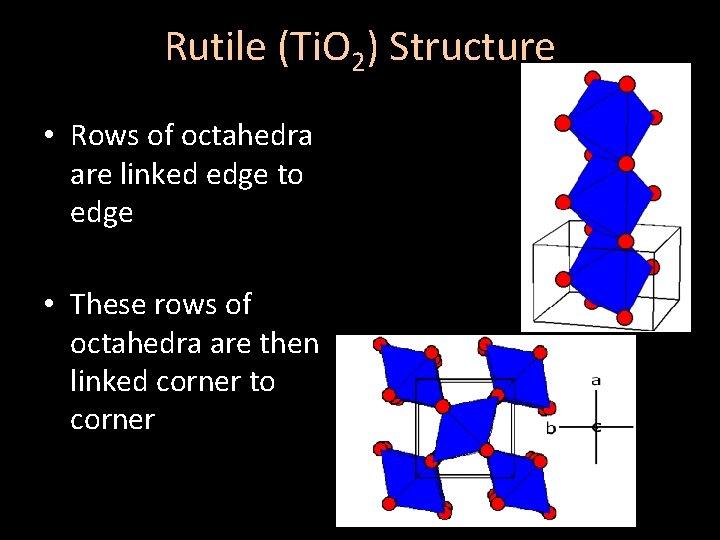
Rutile (Ti. O 2) Structure • Rows of octahedra are linked edge to edge • These rows of octahedra are then linked corner to corner
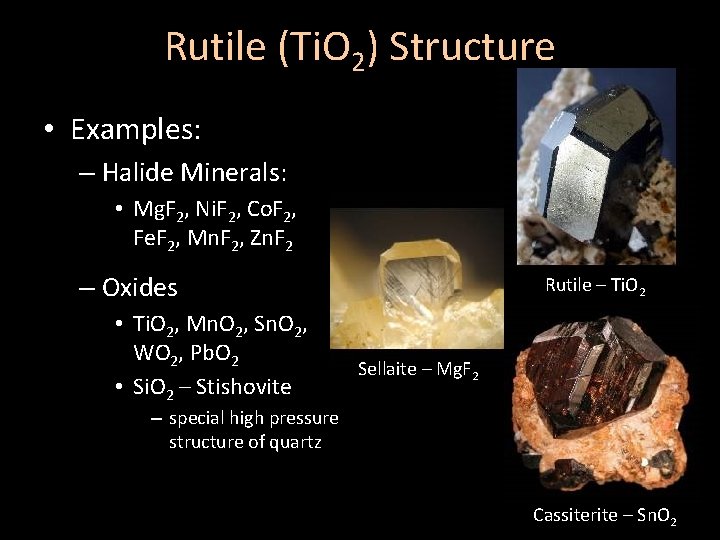
Rutile (Ti. O 2) Structure • Examples: – Halide Minerals: • Mg. F 2, Ni. F 2, Co. F 2, Fe. F 2, Mn. F 2, Zn. F 2 – Oxides • Ti. O 2, Mn. O 2, Sn. O 2, WO 2, Pb. O 2 • Si. O 2 – Stishovite Rutile – Ti. O 2 Sellaite – Mg. F 2 – special high pressure structure of quartz Cassiterite – Sn. O 2
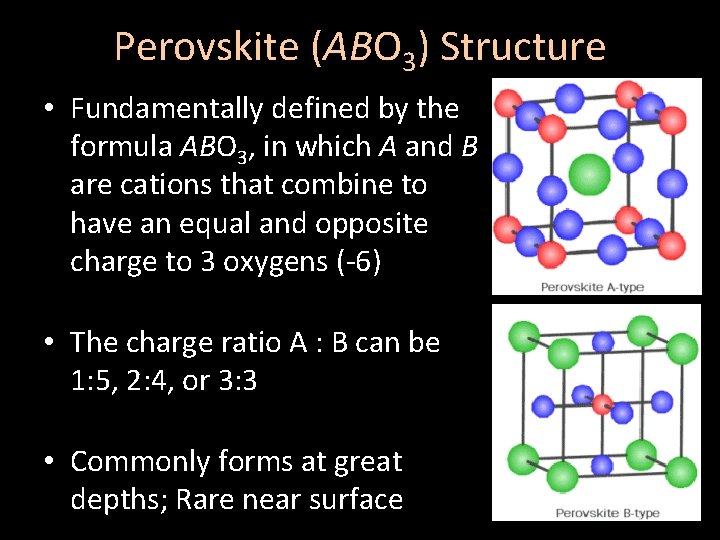
Perovskite (ABO 3) Structure • Fundamentally defined by the formula ABO 3, in which A and B are cations that combine to have an equal and opposite charge to 3 oxygens (-6) • The charge ratio A : B can be 1: 5, 2: 4, or 3: 3 • Commonly forms at great depths; Rare near surface
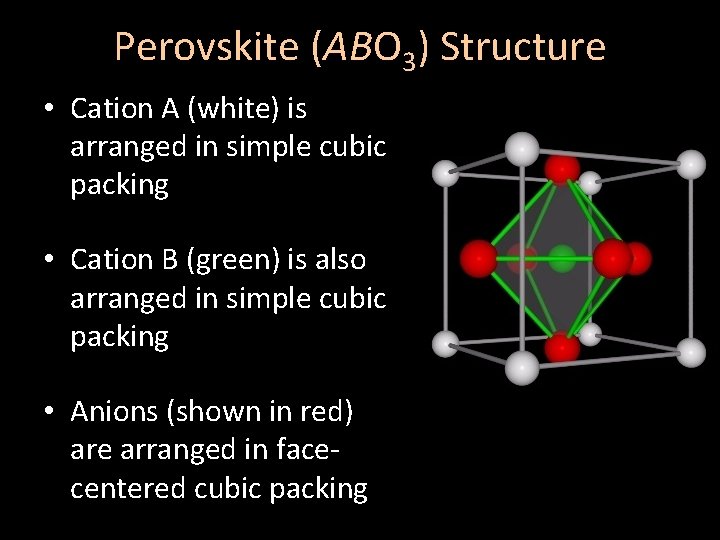
Perovskite (ABO 3) Structure • Cation A (white) is arranged in simple cubic packing • Cation B (green) is also arranged in simple cubic packing • Anions (shown in red) are arranged in facecentered cubic packing
![Perovskite (ABO 3) Structure • Cation A (white) occupies cuboctahedral sites [CN = 12] Perovskite (ABO 3) Structure • Cation A (white) occupies cuboctahedral sites [CN = 12]](http://slidetodoc.com/presentation_image_h2/016635b3ad13ab714f6a81e8291a1565/image-40.jpg)
Perovskite (ABO 3) Structure • Cation A (white) occupies cuboctahedral sites [CN = 12] • Cation B (green) occupies octahedral sites [CN = 6] • Anions (red) occupy octahedral sites [CN = 6]
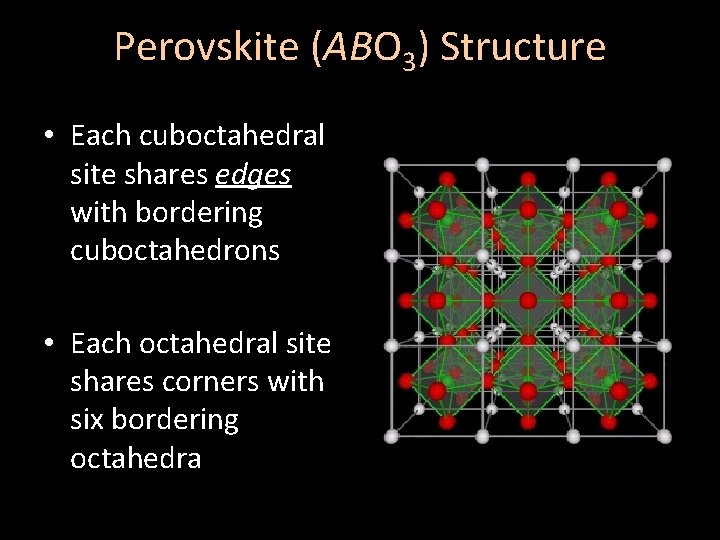
Perovskite (ABO 3) Structure • Each cuboctahedral site shares edges with bordering cuboctahedrons • Each octahedral site shares corners with six bordering octahedra
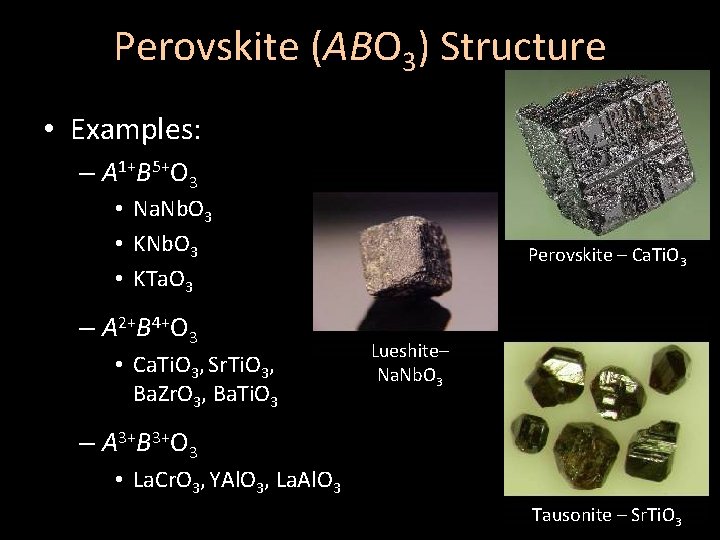
Perovskite (ABO 3) Structure • Examples: – A 1+B 5+O 3 • Na. Nb. O 3 • KTa. O 3 – A 2+B 4+O 3 • Ca. Ti. O 3, Sr. Ti. O 3, Ba. Zr. O 3, Ba. Ti. O 3 Perovskite – Ca. Ti. O 3 Lueshite– Na. Nb. O 3 – A 3+B 3+O 3 • La. Cr. O 3, YAl. O 3, La. Al. O 3 Tausonite – Sr. Ti. O 3
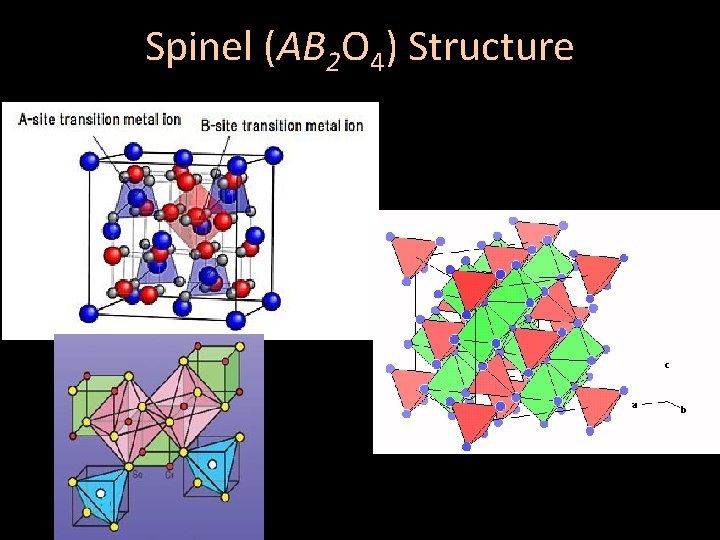
Spinel (AB 2 O 4) Structure
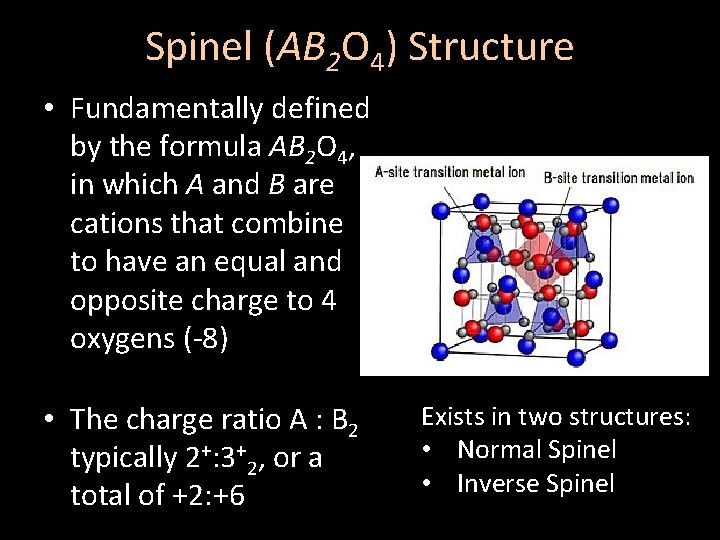
Spinel (AB 2 O 4) Structure • Fundamentally defined by the formula AB 2 O 4, in which A and B are cations that combine to have an equal and opposite charge to 4 oxygens (-8) • The charge ratio A : B 2 typically 2+: 3+2, or a total of +2: +6 Exists in two structures: • Normal Spinel • Inverse Spinel
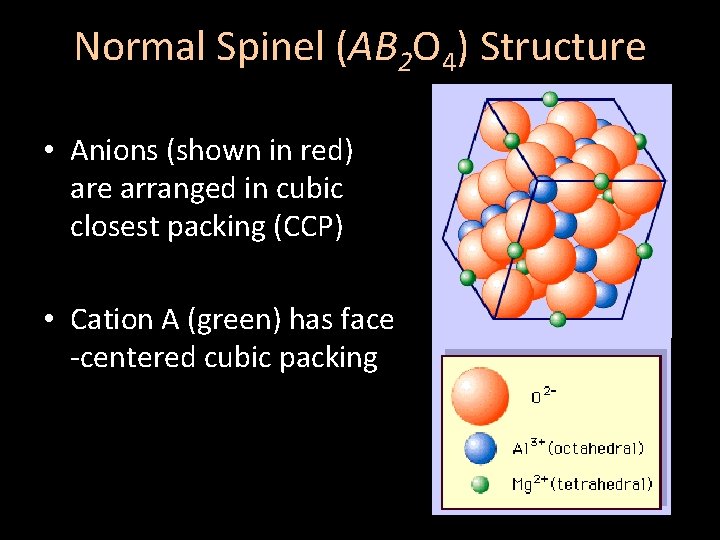
Normal Spinel (AB 2 O 4) Structure • Anions (shown in red) are arranged in cubic closest packing (CCP) • Cation A (green) has face -centered cubic packing
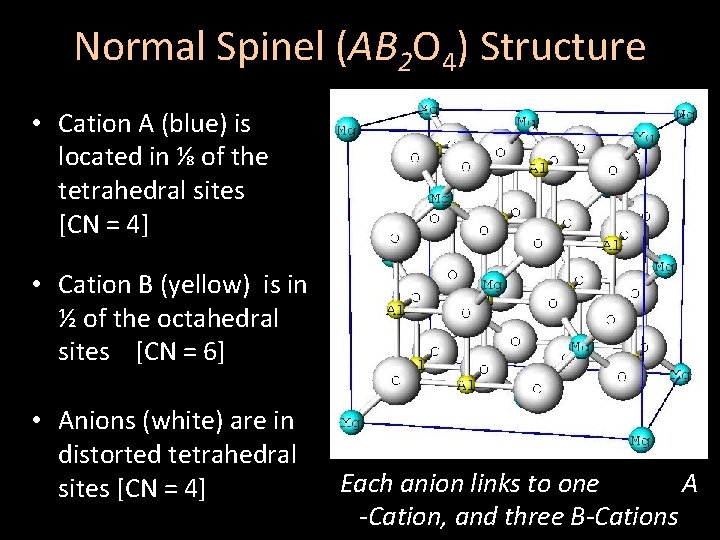
Normal Spinel (AB 2 O 4) Structure • Cation A (blue) is located in ⅛ of the tetrahedral sites [CN = 4] • Cation B (yellow) is in ½ of the octahedral sites [CN = 6] • Anions (white) are in distorted tetrahedral sites [CN = 4] Each anion links to one A -Cation, and three B-Cations
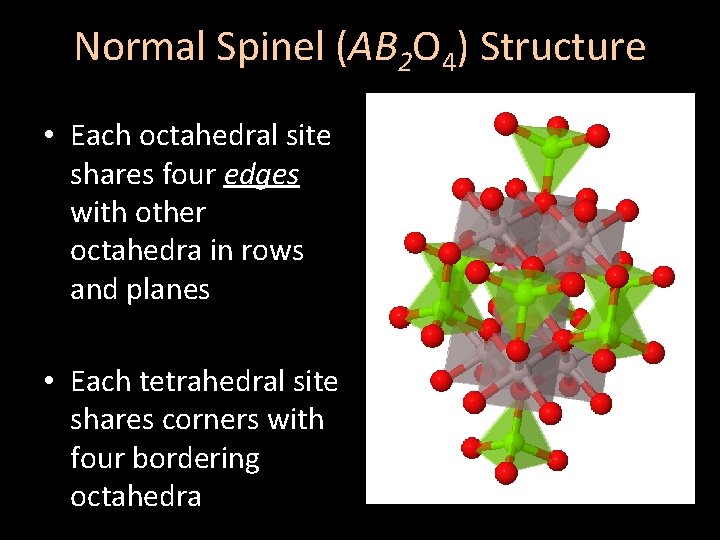
Normal Spinel (AB 2 O 4) Structure • Each octahedral site shares four edges with other octahedra in rows and planes • Each tetrahedral site shares corners with four bordering octahedra
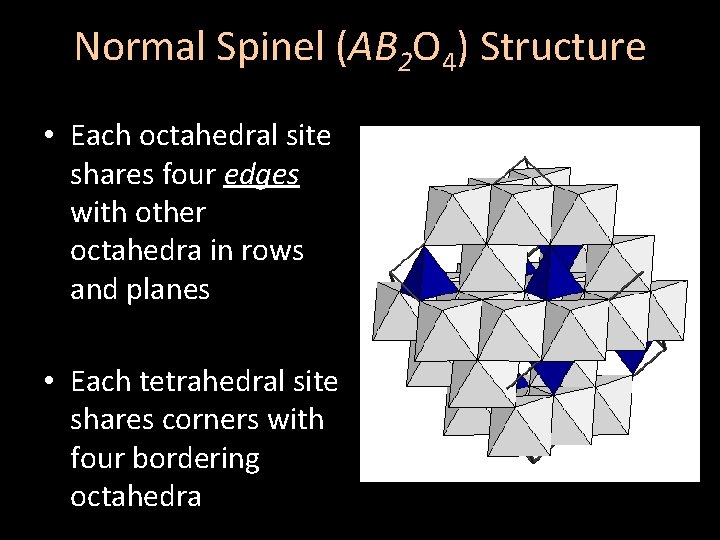
Normal Spinel (AB 2 O 4) Structure • Each octahedral site shares four edges with other octahedra in rows and planes • Each tetrahedral site shares corners with four bordering octahedra
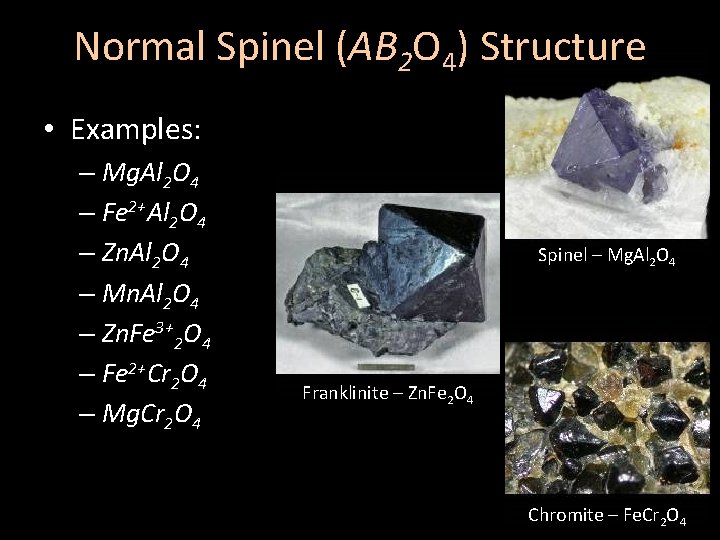
Normal Spinel (AB 2 O 4) Structure • Examples: – Mg. Al 2 O 4 – Fe 2+Al 2 O 4 – Zn. Al 2 O 4 – Mn. Al 2 O 4 – Zn. Fe 3+2 O 4 – Fe 2+Cr 2 O 4 – Mg. Cr 2 O 4 Spinel – Mg. Al 2 O 4 Franklinite – Zn. Fe 2 O 4 Chromite – Fe. Cr 2 O 4
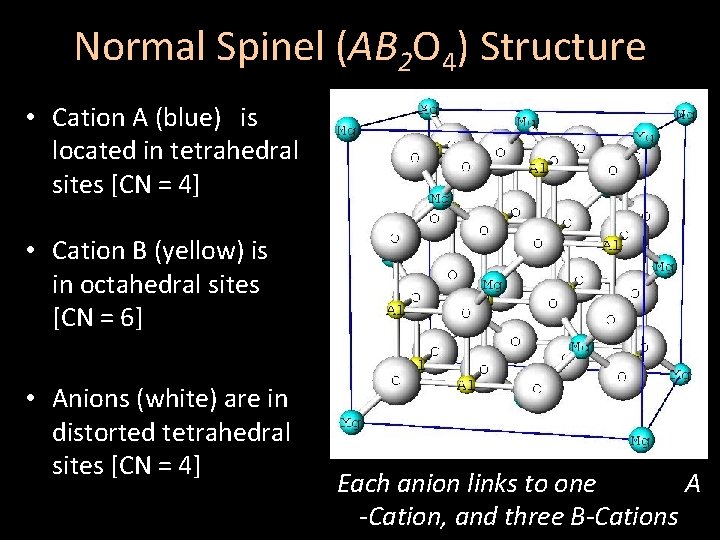
Normal Spinel (AB 2 O 4) Structure • Cation A (blue) is located in tetrahedral sites [CN = 4] • Cation B (yellow) is in octahedral sites [CN = 6] • Anions (white) are in distorted tetrahedral sites [CN = 4] Each anion links to one A -Cation, and three B-Cations
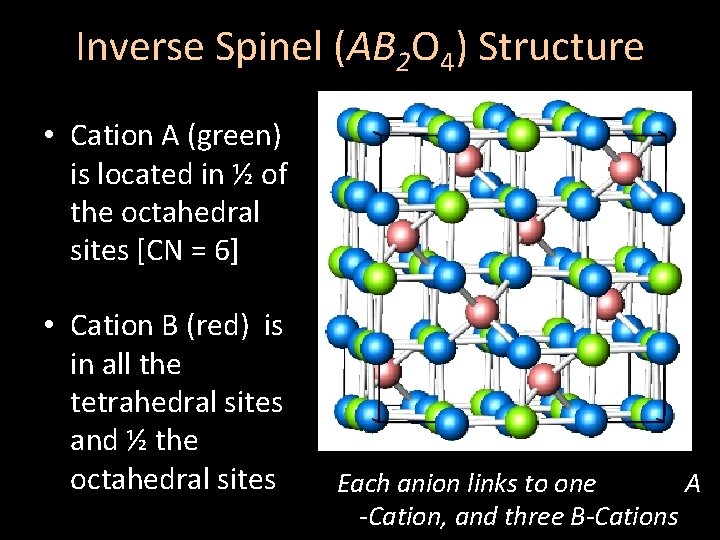
Inverse Spinel (AB 2 O 4) Structure • Cation A (green) is located in ½ of the octahedral sites [CN = 6] • Cation B (red) is in all the tetrahedral sites and ½ the octahedral sites Each anion links to one A -Cation, and three B-Cations
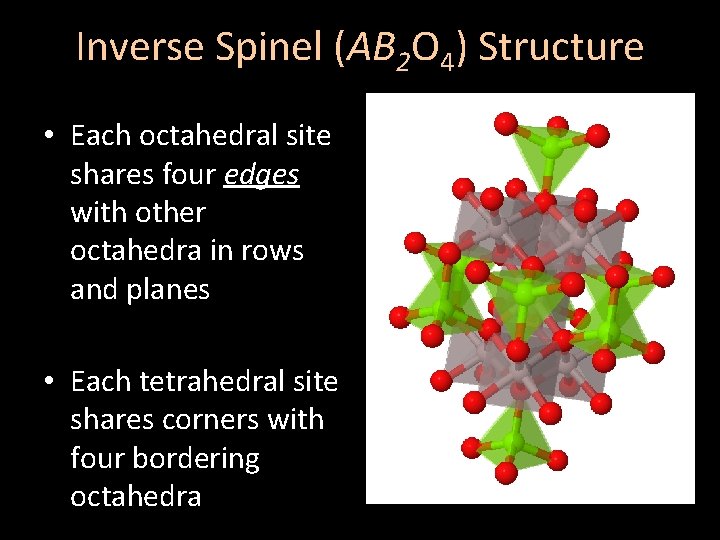
Inverse Spinel (AB 2 O 4) Structure • Each octahedral site shares four edges with other octahedra in rows and planes • Each tetrahedral site shares corners with four bordering octahedra
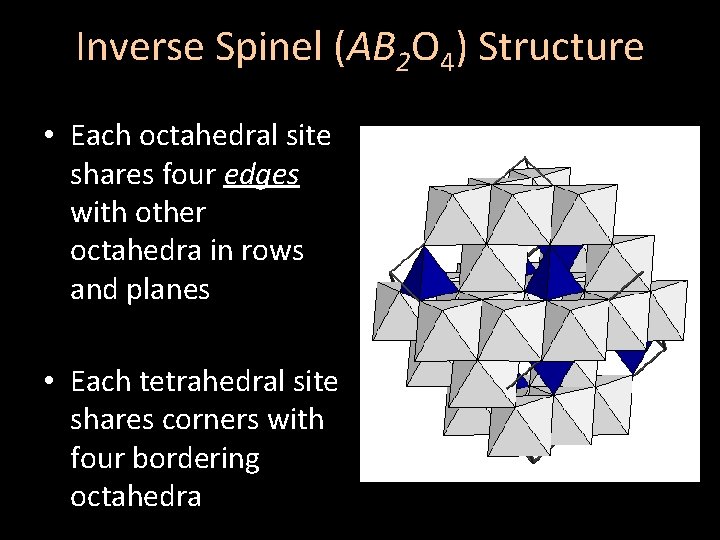
Inverse Spinel (AB 2 O 4) Structure • Each octahedral site shares four edges with other octahedra in rows and planes • Each tetrahedral site shares corners with four bordering octahedra
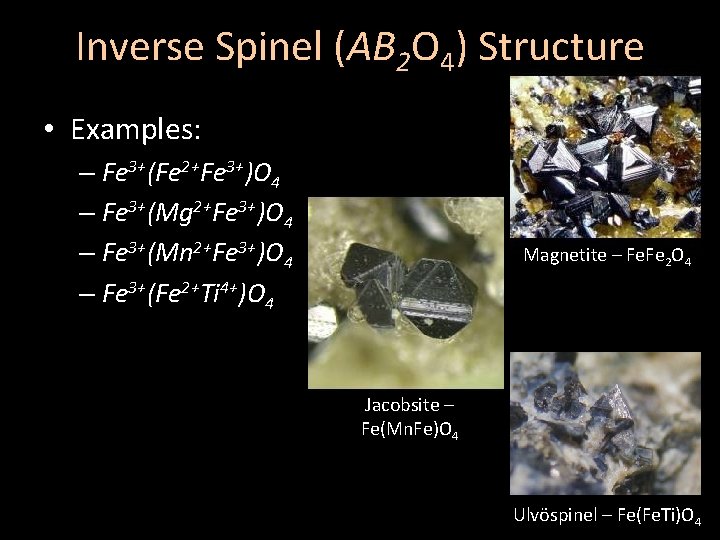
Inverse Spinel (AB 2 O 4) Structure • Examples: – Fe 3+(Fe 2+Fe 3+)O 4 – Fe 3+(Mg 2+Fe 3+)O 4 – Fe 3+(Mn 2+Fe 3+)O 4 – Fe 3+(Fe 2+Ti 4+)O 4 Magnetite – Fe. Fe 2 O 4 Jacobsite – Fe(Mn. Fe)O 4 Ulvöspinel – Fe(Fe. Ti)O 4

Summary: Crystal Structure Basics • Crystal structure affects: – Cleavage – Hardness – Density – Melting Point – Refractive Index – X-Ray Diffraction Pattern – Solid Solution – etc. . .
Summary: Crystal Structure Basics • We derive the sizes of atoms and ions from a crystal structure properties • Determine structure from: – External symmetry – X-ray diffraction effects – Electron diffraction patterns – Density – Chemical composition
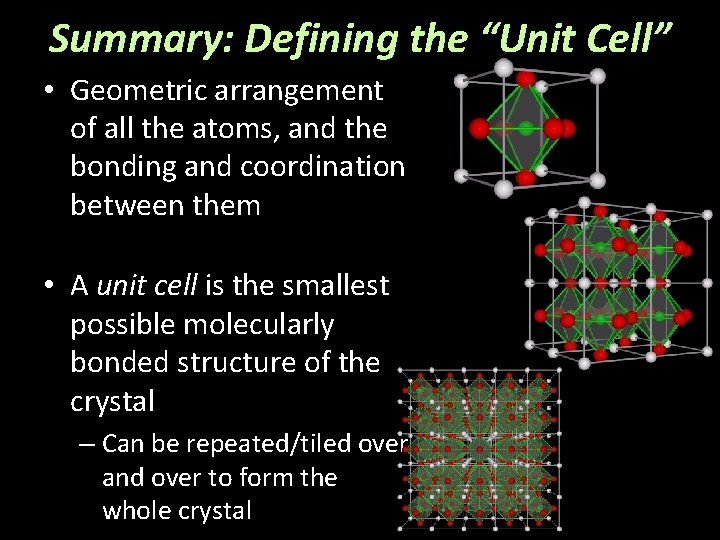
Summary: Defining the “Unit Cell” • Geometric arrangement of all the atoms, and the bonding and coordination between them • A unit cell is the smallest possible molecularly bonded structure of the crystal – Can be repeated/tiled over and over to form the whole crystal
Summary: Isostructuralism • Two crystals or compounds with the same general structure – Does not imply identical properties – hardness, color, melting point, etc – Ex: UO 2 and Ca. F 2 • Not all compounds with same ratio of atoms and charges are isostructural – size effects, etc. – Ex: Ti. O 2 vs. UO 2 – Na. Cl vs. Zn. S
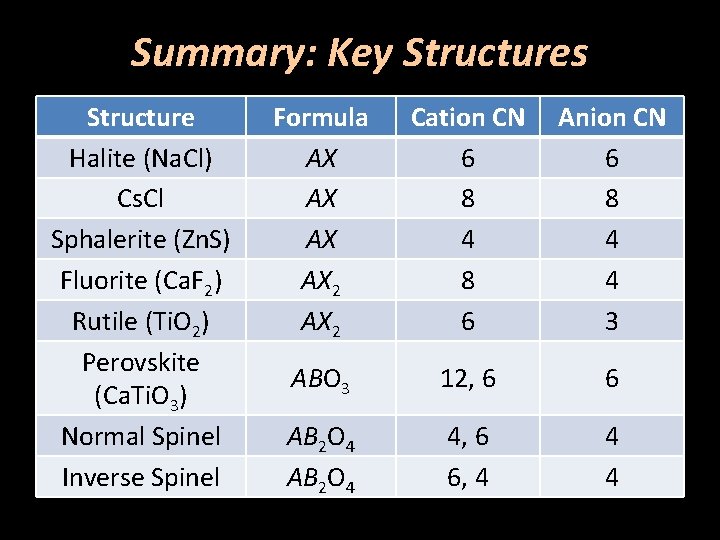
Summary: Key Structures Structure Halite (Na. Cl) Cs. Cl Sphalerite (Zn. S) Fluorite (Ca. F 2) Rutile (Ti. O 2) Perovskite (Ca. Ti. O 3) Normal Spinel Inverse Spinel Formula AX AX 2 Cation CN 6 8 4 8 6 Anion CN 6 8 4 4 3 ABO 3 12, 6 6 AB 2 O 4 4, 6 6, 4 4 4
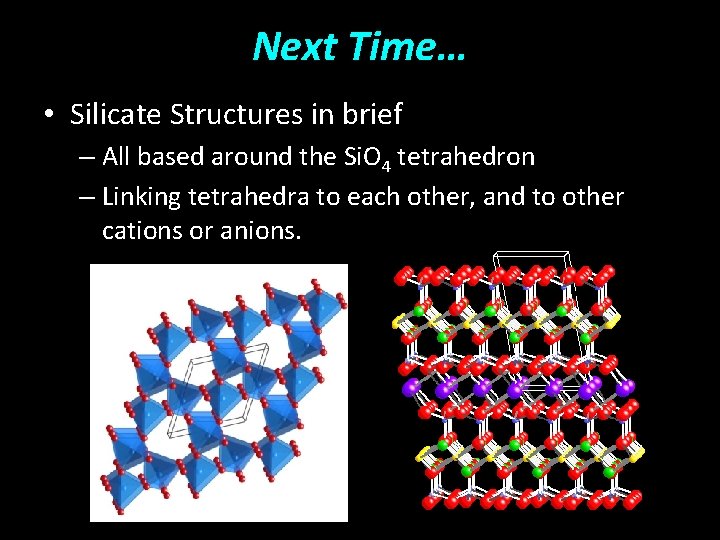
Next Time… • Silicate Structures in brief – All based around the Si. O 4 tetrahedron – Linking tetrahedra to each other, and to other cations or anions.
- Slides: 60