Common Biochemical Reactions chemical reaction a process involving
Common Biochemical Reactions

chemical reaction § a process involving one, two or more substances (called reactants), characterized by a chemical change and yielding one or more product(s) which are different from the reactants
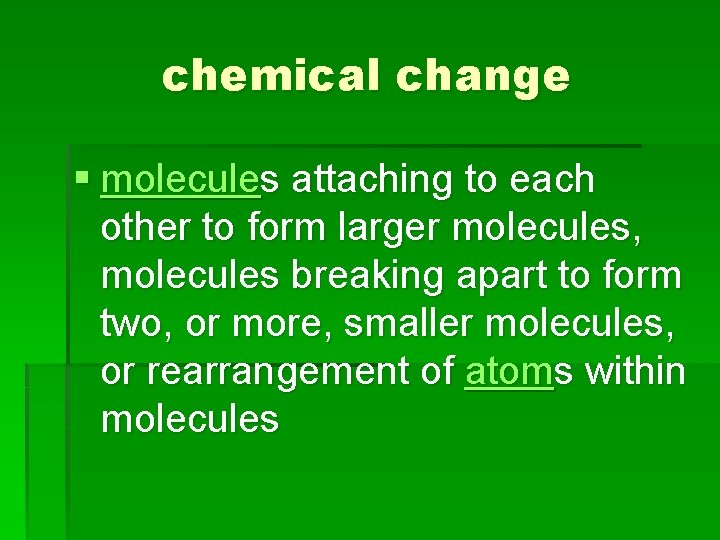
chemical change § molecules attaching to each other to form larger molecules, molecules breaking apart to form two, or more, smaller molecules, or rearrangement of atoms within molecules

chemical change § involve the making or breaking of chemical bonds § does not change the nucleus of the atom in any way, only the interaction of the electron clouds of the involved atoms § Nuclear reactions are not considered chemical reactions, although chemical reactions may follow a nuclear transformation.
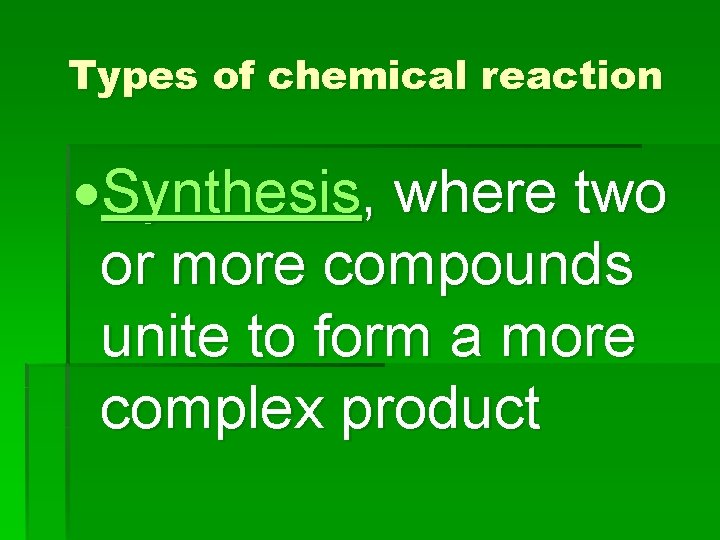
Types of chemical reaction Synthesis, where two or more compounds unite to form a more complex product
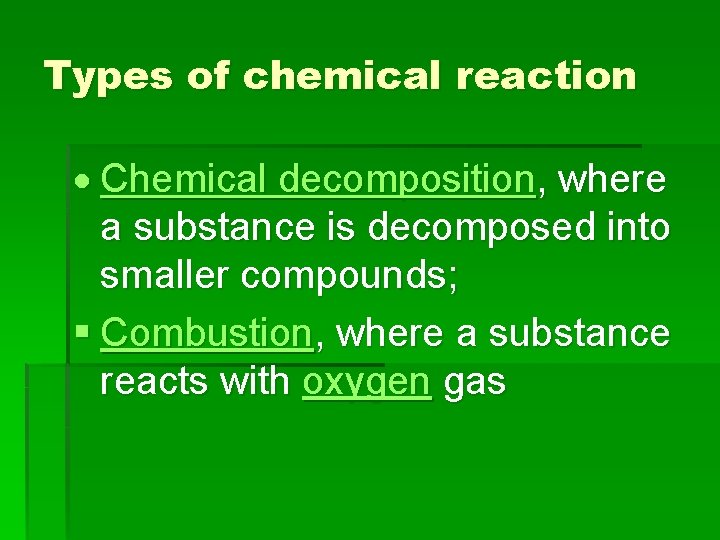
Types of chemical reaction Chemical decomposition, where a substance is decomposed into smaller compounds; § Combustion, where a substance reacts with oxygen gas
Types of chemical reaction Single displacement reaction, also called a single replacement reaction, characterized by an atom of an element which is part of a compound being displaced out of it by a more reactive atom
Types of chemical reaction Double displacement reaction, also called a double replacement reaction, where compounds (usually ionic) exchange ions to form different compounds;

Types of chemical reaction Acid-base neutralization, where an acid an base react producing water;
Types of chemical reaction organic reactions, which encompass several different kinds of reactions involving compounds which have carbon as the main element in their molecular structure.
Types of chemical reaction § redox reactions, which involve electron transference between compounds (metallic single displacement and combustion are two common examples) § acid-base reactions
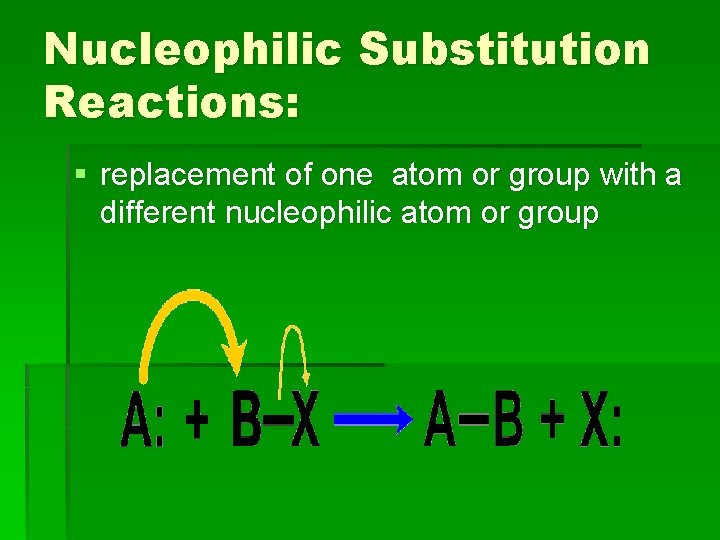
Nucleophilic Substitution Reactions: § replacement of one atom or group with a different nucleophilic atom or group

Nucleophilic Substitution Reactions: §Generally anions or unshared electron pairs

Common biological nucleophiles §oxygen, nitrogen, and sulfur
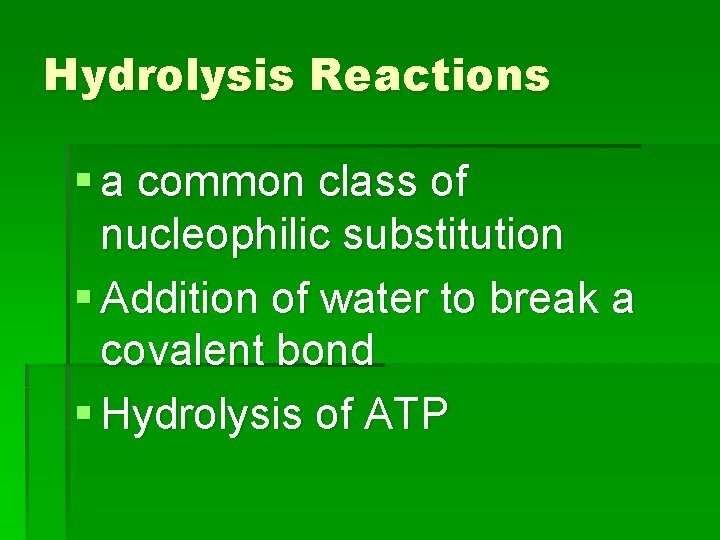
Hydrolysis Reactions § a common class of nucleophilic substitution § Addition of water to break a covalent bond § Hydrolysis of ATP

Elimination Reactions § removal of atoms to form a double bond § Dehydration: removal of water (e. g. from a C - C bond to form a C = C double bond

Addition Reactions § combining two molecules to form a single product (generally across a C = C double bond) § Hydration: Addition of water (e. g. to a C = C double bond) § Ex. a late step in the Krebs Cycle

Isomerization Reactions § intramolecular rearrangement of atoms or groups (e. g. isomerization of sugar molecules

Oxidation-Reduction Reactions § transfer of electrons from a donor (reducing agent) to an acceptor molecule (oxidizing agent) § Reduction: gain of electrons § gain of Hydrogen or loss of Oxygen § Oxidation: loss of electrons § gain of Oxygen or loss of Hydrogen § Note: Oxidation and Reduction are coupled reactions (REDOX)
- Slides: 19