Commissioning comparative effectiveness research CER Cochrane Skin Group































- Slides: 46

Commissioning comparative effectiveness research (CER) Cochrane Skin Group Meeting, Denver, 2010 Hywel Williams, Chair of the NIHR HTA Commissioning Board 9/18/2020

What I am going to do: • Definitions – what is CER and what is it not? • Something about CER in the US • Commissioning CER in the UK

1. Definitions – US Health and Human Services • Comparative effectiveness research is the conduct and synthesis of research comparing the benefits and harms of different interventions and strategies to prevent, diagnose, treat and monitor health conditions in “real world” settings. • The purpose of this research is to improve health outcomes by developing and disseminating evidence makers, responding to their expressed needs, about which interventions are most effective for which patients under specific circumstances. Source: Anne Trontell. AHRQ, April 2010 http: //www. fda. gov/downloads/Drugs/News. Events/UCM 209104. pdf

More about the spirit of CER • Seeks to improve health care and outcomes by providing evidence-based information that clinicians, patients, and policymakers want • Compares real world benefits and harms, especially in understudied populations and subgroups • Includes all therapies and comparisons, ie drug vs. device vs. procedure • Conducts and synthesizes research – Experimental, observational, analytical • Disseminates information for independent decisions and actions by multiple stakeholders

CER is NOT: • Solely about effectiveness • Solely about cost-effectiveness • Intended as regulatory or directive • Restricted to randomized controlled trials • Exclusionary of clinical judgment or the circumstances of the individual patient • Aimed at limiting or restricting health services

2. CER in the US – massive new investment ARRA, the American Recovery and Reinvestment Act of 2009 included $1. 1 billion for comparative effectiveness research: • AHRQ: $300 million • NIH: $400 million • Secretary’s Office of the Secretary: $400 million (allocated at the Secretary’s discretion)





3. Back to the UK. . . .

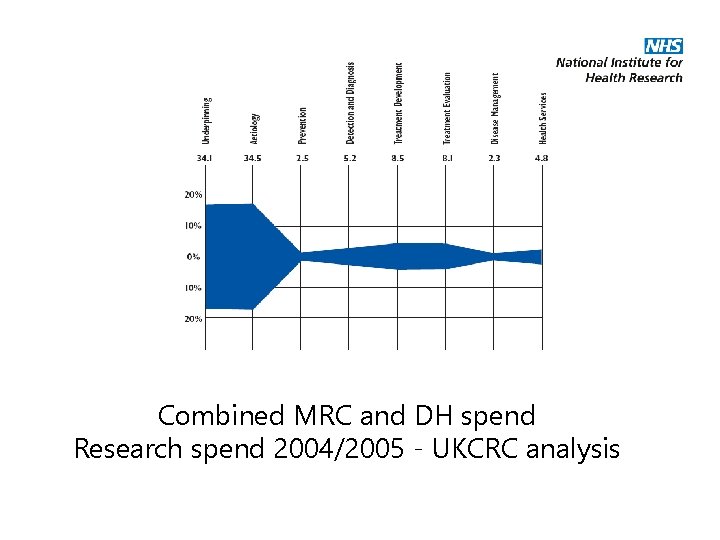
Combined MRC and DH spend Research spend 2004/2005 - UKCRC analysis

Key issues that needed addressing • Decline in clinical research community • Decline in infrastructure for clinical research • Complex regulatory environment • Need to recognise Industry R&D needs in the UK • Not yet realising the Potential of a single National Health Service

NHS R&D Strategy “To create a health research system in which the NHS supports outstanding individuals, working in world-class facilities, conducting leading-edge research, focused on the needs of patients and the public”

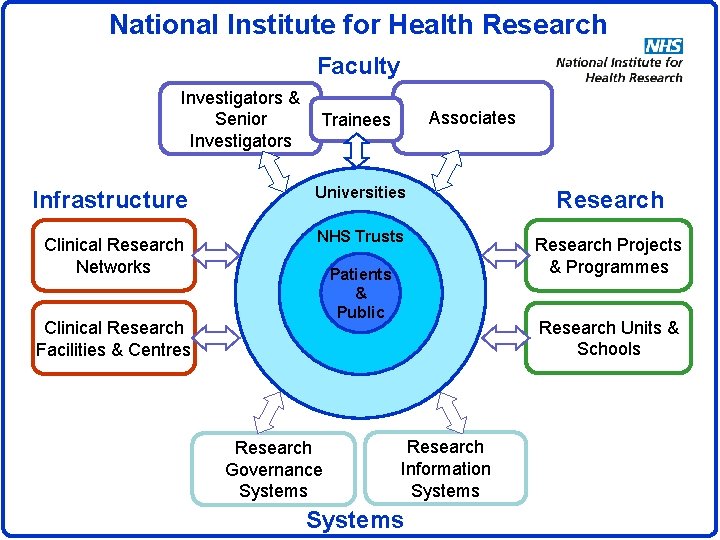
National Institute for Health Research Faculty Investigators & Senior Investigators Associates Trainees Infrastructure Universities Research Clinical Research Networks NHS Trusts Research Projects & Programmes Patients & Public Clinical Research Facilities & Centres Research Governance Systems Research Units & Schools Research Information Systems

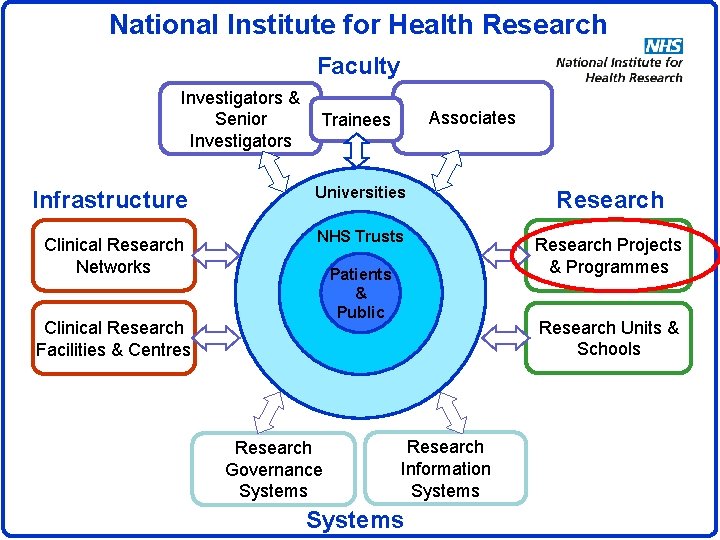
National Institute for Health Research Faculty Investigators & Senior Investigators Associates Trainees Infrastructure Universities Research Clinical Research Networks NHS Trusts Research Projects & Programmes Patients & Public Clinical Research Facilities & Centres Research Governance Systems Research Units & Schools Research Information Systems

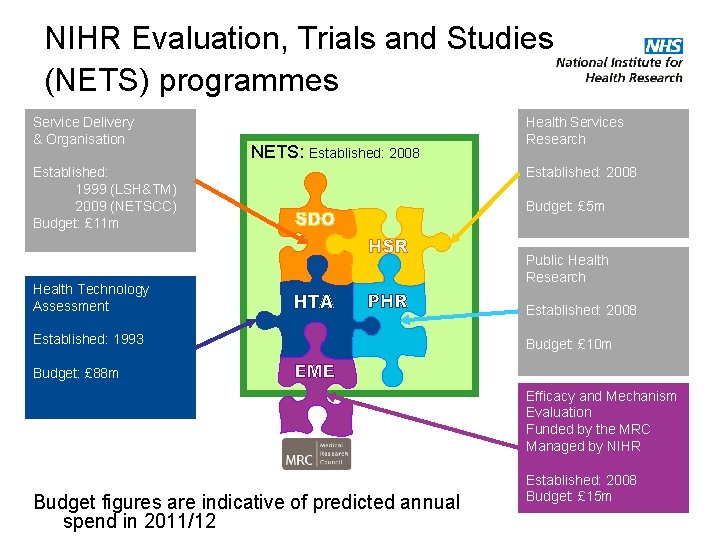
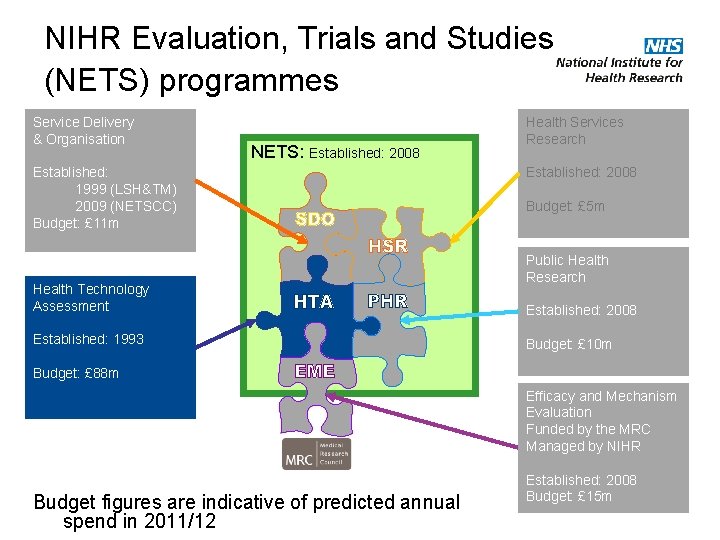
NIHR Evaluation, Trials and Studies (NETS) programmes Service Delivery & Organisation NETS: Established: 2008 Established: 1999 (LSH&TM) 2009 (NETSCC) Budget: £ 11 m Health Technology Assessment Established: 1993 Health Services Research Established: 2008 Budget: £ 5 m Public Health Research Established: 2008 Budget: £ 10 m Budget: £ 88 m Efficacy and Mechanism Evaluation Funded by the MRC Managed by NIHR Budget figures are indicative of predicted annual spend in 2011/12 Established: 2008 Budget: £ 15 m

Efficacy and Mechanism Evaluation (EME) programme • Remit To support clinical trials and studies which: – add significantly to our understanding of biological or behavioural mechanisms and processes; – explore new scientific or clinical principles; – evaluate clinical efficacy of healthcare interventions (drugs, technology, diagnostics, procedures) • Laboratory studies as part of the main study • Include validated surrogate markers as indicators of outcome • Responsive mode – “pull through”

EME does not support: • Incremental modifications • Refinements of existing technologies • Proof of concept • Proof of mechanism in human • Confidence in Effect • Very early phase Clinical Trials (I, IIa) www. eme. ac. uk

The Managed Translational Pathway

The Managed Translational Pathway Successful development? “Pull through”

The Managed Translational Pathway Promising, non commercial

The Managed Translational Pathway Fail in testing, no longer promising, or commercial partner

NIHR Evaluation, Trials and Studies (NETS) programmes Service Delivery & Organisation NETS: Established: 2008 Established: 1999 (LSH&TM) 2009 (NETSCC) Budget: £ 11 m Health Technology Assessment Established: 1993 Health Services Research Established: 2008 Budget: £ 5 m Public Health Research Established: 2008 Budget: £ 10 m Budget: £ 88 m Efficacy and Mechanism Evaluation Funded by the MRC Managed by NIHR Budget figures are indicative of predicted annual spend in 2011/12 Established: 2008 Budget: £ 15 m

Getting innovations into practice Does it work? Is it safe? Can it be allowed to be done in the NHS? What if it is done in the NHS? Horizon-scanning Regulation (MHRA, Should it be done in the NHS appraisal IPAC-NICE) Basic biomedical research MRC, Various funders Translational research Safety and efficacy MRC, NEAT Effectiveness and cost-effectiveness HTA General clinical use SDO Appraisal (NICE) EME Specialist commissioning

Tasks for the HTA Programme • Identifying needs of NHS for research into technologies • What are the large and challenging problems? • Who else will examine them? • Getting the right questions at the right time • Commissioning/monitoring research • Getting timely and useful results to decision-makers – To allow them to act on the answers • The programme is: – Needs- led – relevance to the NHS – Science- added – seeks to add value at every stage

1993 2006 2005 1999 2006

Commissioned research – us pulling the community to do “dull but needed” research

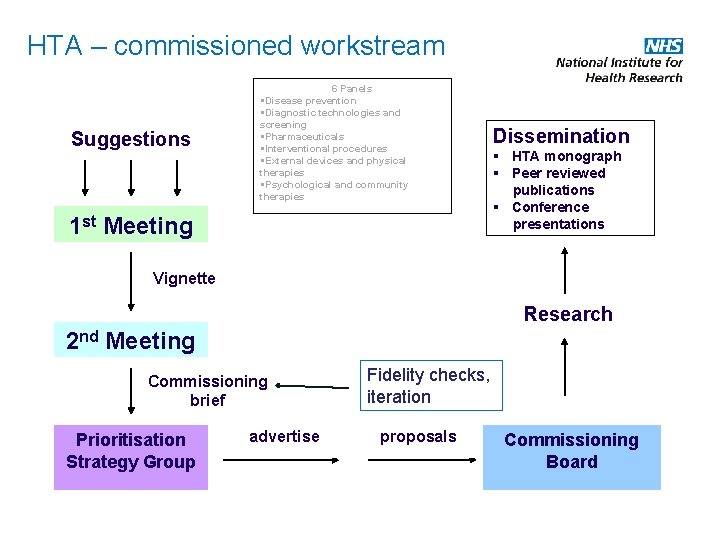
HTA – commissioned workstream Suggestions 6 Panels §Disease prevention §Diagnostic technologies and screening §Pharmaceuticals §Interventional procedures §External devices and physical therapies §Psychological and community therapies 1 st Meeting Dissemination § HTA monograph § Peer reviewed publications § Conference presentations Vignette Research 2 nd Meeting Commissioning brief Prioritisation Strategy Group advertise Fidelity checks, iteration proposals Commissioning Board

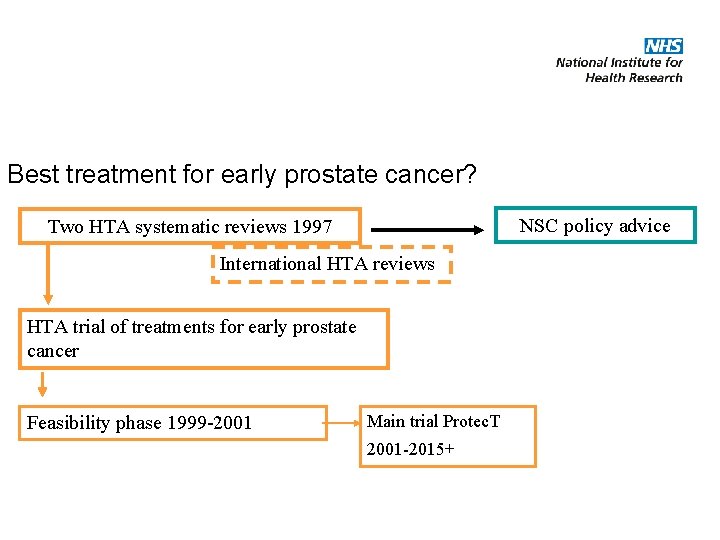
Best treatment for early prostate cancer? NSC policy advice Two HTA systematic reviews 1997 International HTA reviews HTA trial of treatments for early prostate cancer Feasibility phase 1999 -2001 Main trial Protec. T 2001 -2015+

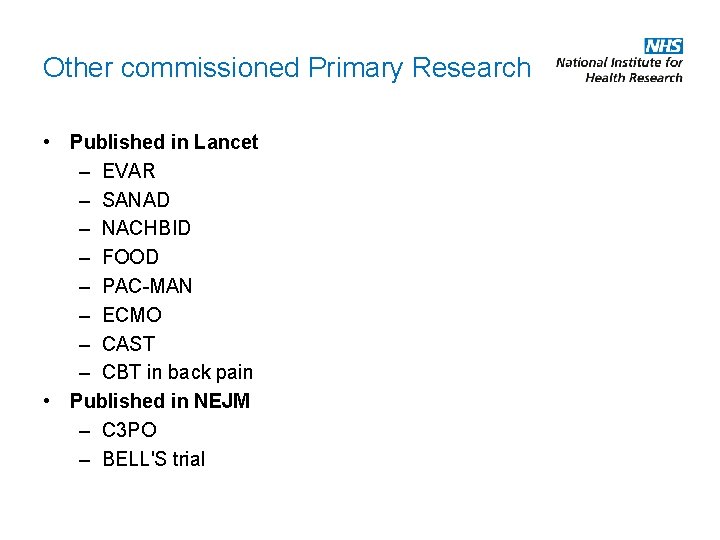
Other commissioned Primary Research • Published in Lancet – EVAR – SANAD – NACHBID – FOOD – PAC-MAN – ECMO – CAST – CBT in back pain • Published in NEJM – C 3 PO – BELL'S trial

1993 2006 2005 1999 2006

Responsive mode – research community pulling us

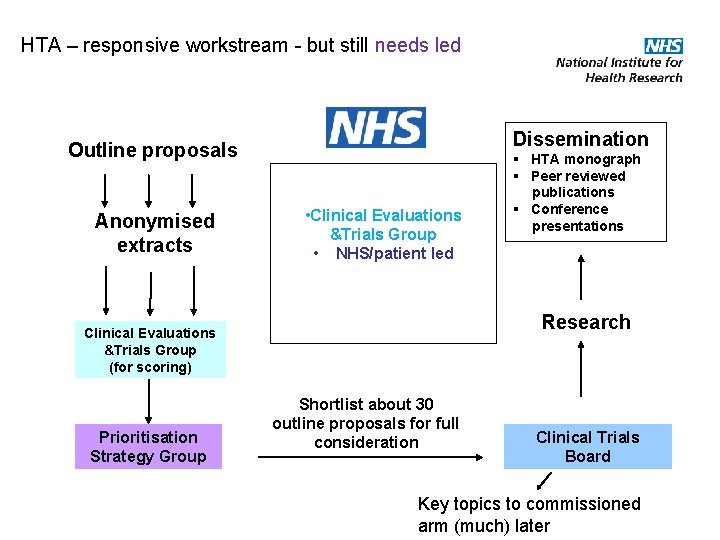
HTA – responsive workstream - but still needs led Dissemination Outline proposals Anonymised extracts • Clinical Evaluations &Trials Group • NHS/patient led Research Clinical Evaluations &Trials Group (for scoring) Prioritisation Strategy Group § HTA monograph § Peer reviewed publications § Conference presentations Shortlist about 30 outline proposals for full consideration Clinical Trials Board Key topics to commissioned arm (much) later

Examples of responsive mode • Themed calls – M 4 C, trauma & emergency care, healthcare acquired infections, – diagnostics – Mental health, stroke • IVAN – bevicizumab v ranibizumab – Inhibit VEGF in Age-related choroidal Neovascularisation. • Persephone - comparing six months Trastuzumab treatment with twelve months, in women with early stage breast cancer

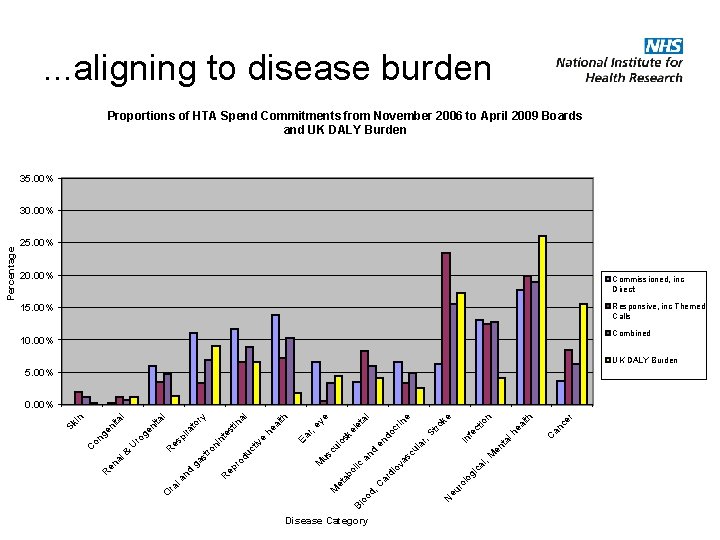
. . . aligning to disease burden Proportions of HTA Spend Commitments from November 2006 to April 2009 Boards and UK DALY Burden 35. 00% 20. 00% Commissioned, inc Direct Responsive, inc Themed Calls 15. 00% Combined 10. 00% UK DALY Burden 5. 00% Disease Category ca l, gi lo ro eu N er an c C lth M en ta lh ea r, In fe St ct ro io n ke e la cu di ar d, C Bl oo M et ab o ov lic as an d cu lo en d sk oc el et rin al e ey M us e iv du ct ro ep R Ea al he es nt ni ro st r, th al tin ry to ira ga O ra la R nd en al & es p U ro ge ge ni on C R in ta l ni ta l 0. 00% Sk Percentage 30. 00%

HTA dermatology related trials • Antibiotics for acne - Topical benzoyl peroxide and benzoyl peroxide/erythromycin combinations are similar in efficacy to oral oxytetracycline and minocycline and are not affected by propionibacterial antibiotic resistance • Softened water for eczema study (SWET) • The Bullous Pemphigoid Steroids and Tetracyclines Study (BLISTER) Ozolins M et al Comparison of five antimicrobial regimens for treatment of mild to moderate inflammatory facial acne vulgaris in the community: randomised controlled trial. Lancet. 2004 Dec 18 -31; 364(9452): 2188 -95.

NETS as a system Facilitating researchers, speeding review Transfers between NETS programmes Active collaboration between programmes Directors' meetings Joint calls Meeting with networks

Right infrastructure and capacity • • • Range of researchers Clinical trials units and research design services Research embedded in service UK Clinical Research Networks Efficient and appropriate R&D governance and ethics

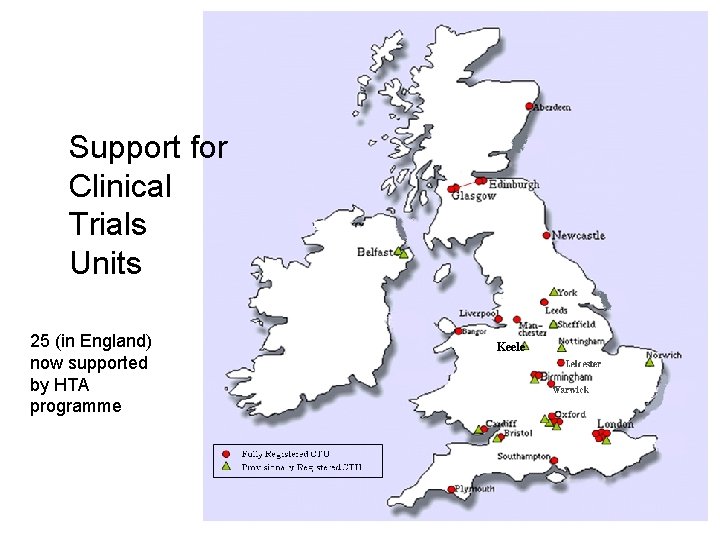
Support for Clinical Trials Units 25 (in England) now supported by HTA programme Keele

How many clinical trials? Other schemes NIHR Rf. PB programme grants Public health SDO MRC DCS


Take-home Messages 1. The clinical research landscape in the UK has been transformed (and very rapidly) 2. . but more to be done 3. There is a greatly increased focus on translational, clinical and applied health research with unprecedented opportunities for those wishing to conduct this research 4. EME and HTA are the vehicles for larger trials

What I have covered: • Definitions – what is CER and what is it not? • Something about CER in the US • Commissioning CER in the UK

Getting the balance right between pushing and pulling research HTA
