Combustion reactions Things go boom Whoosh Jug Combustion

Combustion reactions Things go boom
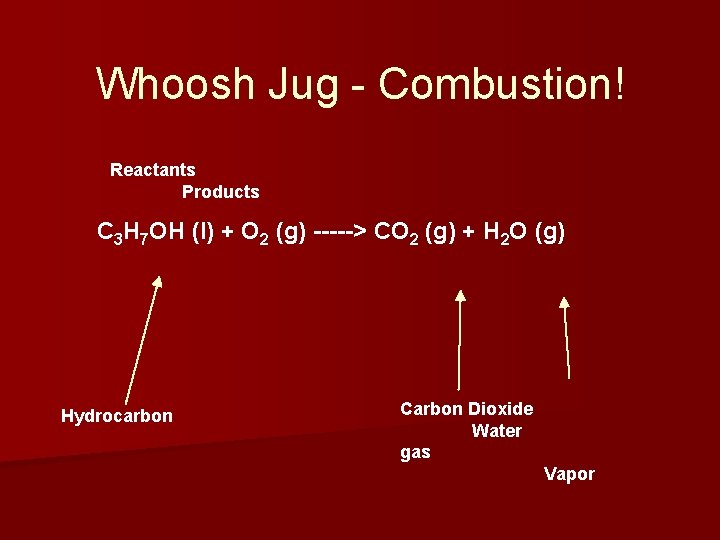
Whoosh Jug - Combustion! Reactants Products C 3 H 7 OH (l) + O 2 (g) -----> CO 2 (g) + H 2 O (g) Hydrocarbon Carbon Dioxide Water gas Vapor
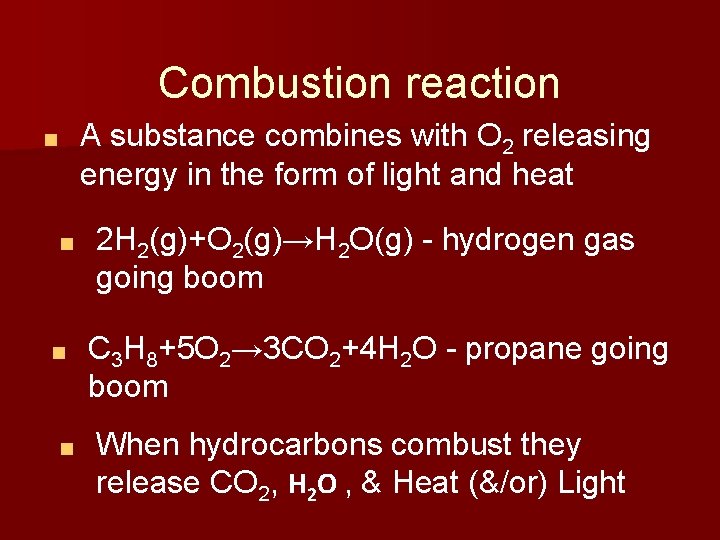
Combustion reaction A substance combines with O 2 releasing energy in the form of light and heat ■ ■ 2 H 2(g)+O 2(g)→H 2 O(g) - hydrogen gas going boom C 3 H 8+5 O 2→ 3 CO 2+4 H 2 O - propane going boom When hydrocarbons combust they release CO 2, H 2 O , & Heat (&/or) Light

Is similar to Redox (next week) ■ The oxygen is so electronegative it pulls electron density away from the fuel, leading to an oxidation of the fuel. ■ Oxidation means loses electrons, reduction means gains electrons.
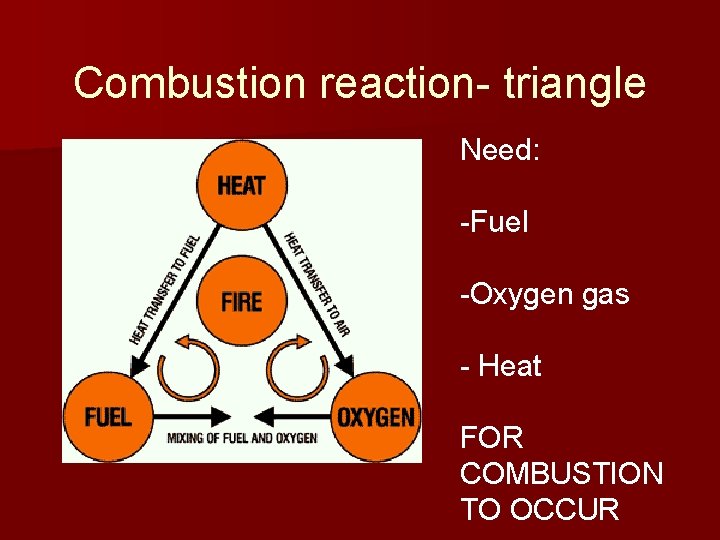
Combustion reaction- triangle Need: -Fuel -Oxygen gas - Heat FOR COMBUSTION TO OCCUR
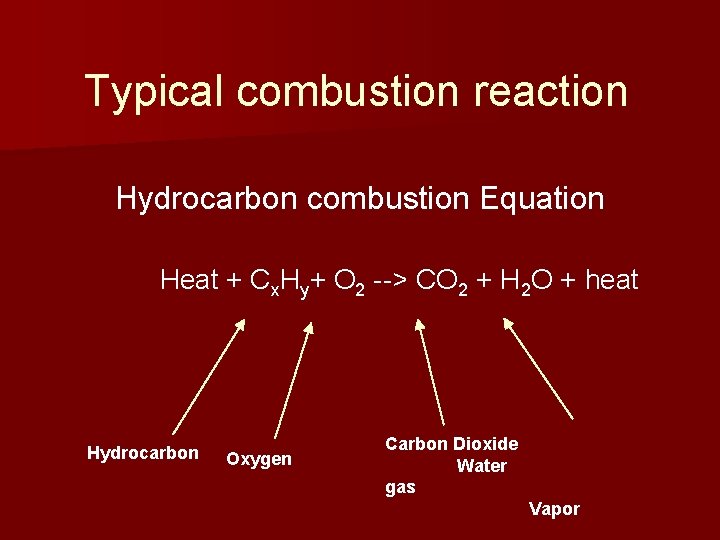
Typical combustion reaction Hydrocarbon combustion Equation Heat + Cx. Hy+ O 2 --> CO 2 + H 2 O + heat Hydrocarbon Oxygen Carbon Dioxide Water gas Vapor
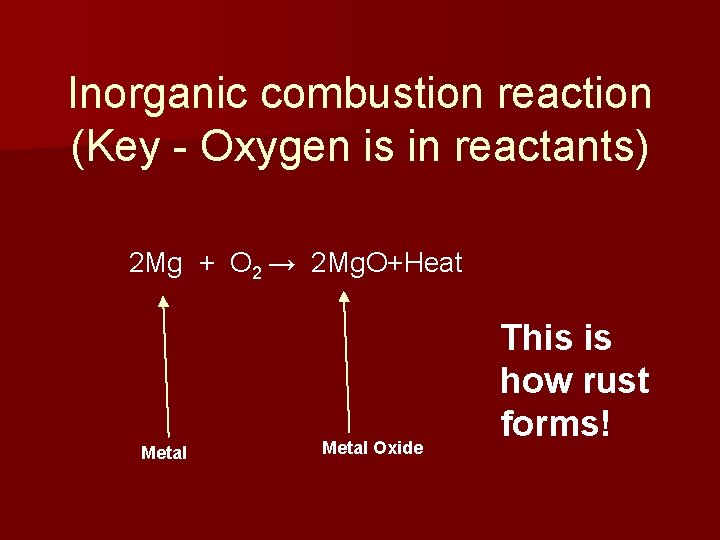
Inorganic combustion reaction (Key - Oxygen is in reactants) 2 Mg + O 2 → 2 Mg. O+Heat Metal Oxide This is how rust forms!

Summarize ■ Explosions occur because a chemical reaction called combustion has taken place. In combustion, oxygen reacts with a substance such as fuel. The process of rusting, in which iron oxide in a metal reacts with oxygen from the air, is an example of slow combustion. Rapid combustion results when there is a rapid release of heat. If the release of heat and gas is extremely rapid, and the gas cannot dissipate quickly enough, then extremely rapid combustion and explosions occur.
- Slides: 8