Combustion Reactions In this lesson you will learn

Combustion Reactions

In this lesson you will learn • to write balanced equations for both complete and incomplete combustion reactions • define exothermic and endothermic reactions • draw energy diagrams representing exothermic and endothermic reactions



Hydrocarbon Combustion • We will be examining combustion (burning) of Hydrocarbons!!

What are Hydrocarbons? • Hydrocarbons – Substances that consist of hydrogen, (H) and carbon, (C) atoms ONLY. • Propane - C 3 H 8 • Octane - C 8 H 18 • Candle wax C 25 H 52
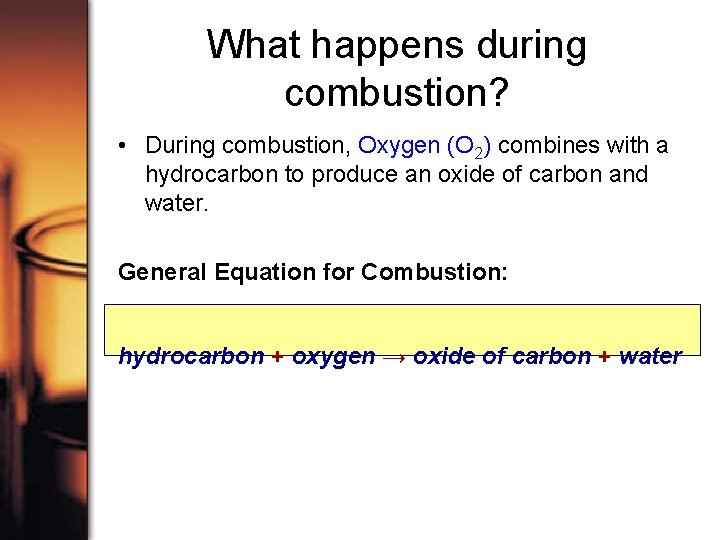
What happens during combustion? • During combustion, Oxygen (O 2) combines with a hydrocarbon to produce an oxide of carbon and water. General Equation for Combustion: hydrocarbon + oxygen → oxide of carbon + water

There are TWO types of Hydrocarbon Combustions. Complete Combustion: • Reaction evidence is the presence of a nice blue flame – clean burning hydrocarbon + oxygen → carbon dioxide + water

Incomplete Combustion: • Carbon monoxide and carbon in the form of soot are produced when combustion is incomplete resulting in an orange, smoky flame. hydrocarbon + oxygen → carbon monoxide + carbon + water

Writing and Balancing Combustion Reactions • A great method to use when balancing combustion reactions is The Method of C H O. That is: 1. balance C 2. balance H 3. balance O last. Example: Balance the complete combustion reaction of Propane (C 3 H 8). ___C 3 H 8(g) + ____ O 2(g) ___CO 2(g) + ___ H 2 O(g)

Write a Balanced Chemical equation for the following situation: A propane fireplace malfunctions and burns its gas incompletely to produce deadly carbon monoxide gas and water.

a. Write a balanced chemical equation for the complete combustion of Pentane, C 5 H 12

Endothermic and Exothermic Reactions • These are reactions that involve ENERGY!! • MOST ALL reactions are EITHER endothermic or exothermic.

Exothermic Reactions • Any reaction which releases energy is said to be exothermic. – Examples: • • • Burning/combustion “hot packs” Digestion of food Bombs exploding Adding group 1 metals to water

Endothermic Reactions • Any reaction in which energy is absorbed is said to be endothermic. – Examples: • Adding Ammonium Nitrate to water. • "Cold Packs" used by athletes contain chemicals which absorb heat from the body.

Energy Diagrams • These are diagrams that show what happens to the energy in a reaction. • There are TWO types of Energy Diagrams.

Energy Diagrams for EXOTHERMIC Reactions Energy NOTE: Energy of Products is LESS than the energy of the Reactants. ENERGY IS LOST!!!

Energy Diagram for Endothermic Reactions Energy NOTE: Energy of Products is GREATER than the energy of the Reactants. ENERGY IS GAINED!!!
- Slides: 18