Combustion Calculation 165472 Power Plant Engineering Combustion Rapid

Combustion Calculation 165472 Power Plant Engineering

Combustion “Rapid oxidation generating heat, or both light and heat; also, slow oxidation accompanied by relatively little heat and no light” (from Webster’s Dictionary) For our purpose, consider only rapid oxidation.

Reactant and Product Mixtures Stoichiometry – Quantity of oxidizer is just that amount needed to completely burn a quantity of fuel. – The most common oxidizer is air Oxidizer > Stoichiometric oxidizer – Fuel lean or lean Oxidizer < Stoichiometric oxidizer – Fuel rich or rich
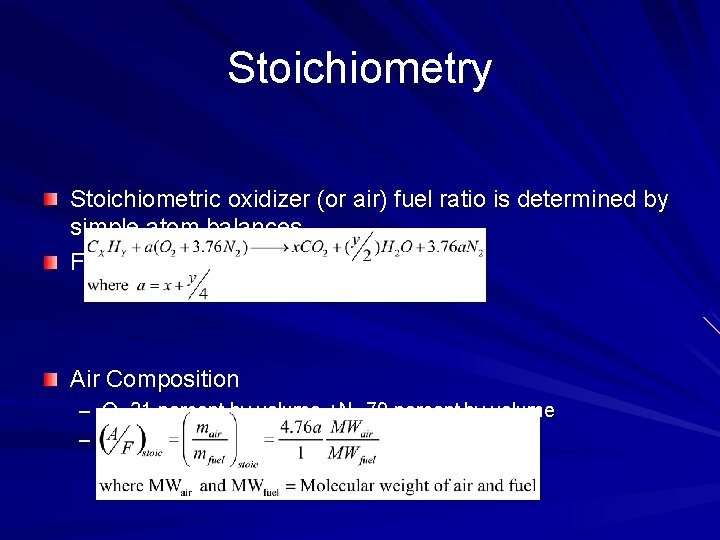
Stoichiometry Stoichiometric oxidizer (or air) fuel ratio is determined by simple atom balances. For hydrocarbon Cx. Hy Air Composition – O 2 21 percent by volume +N 2 79 percent by volume – Mole O 2 : Mole N 2 = 1 : 3. 76
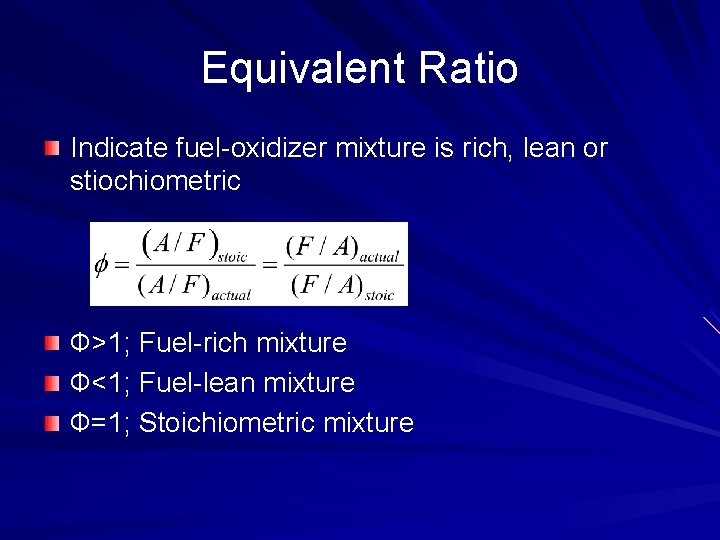
Equivalent Ratio Indicate fuel-oxidizer mixture is rich, lean or stiochiometric Ф>1; Fuel-rich mixture Ф<1; Fuel-lean mixture Ф=1; Stoichiometric mixture

Excess Air Stoichiometric air Excess air

Example 1 A small low-emission stationary gasturbine engine operates at full load (3, 950 k. W) at an equivalent ratio of 0. 286 with an air flowrate of 15. 9 kg/s. The equivalent composition of the fuel (natural gas) is C 1. 16 H 4. 32. Determine the fuel mass flowrate and the operating air-fuel ratio for the engine.

Low NOx gas turbine combustor
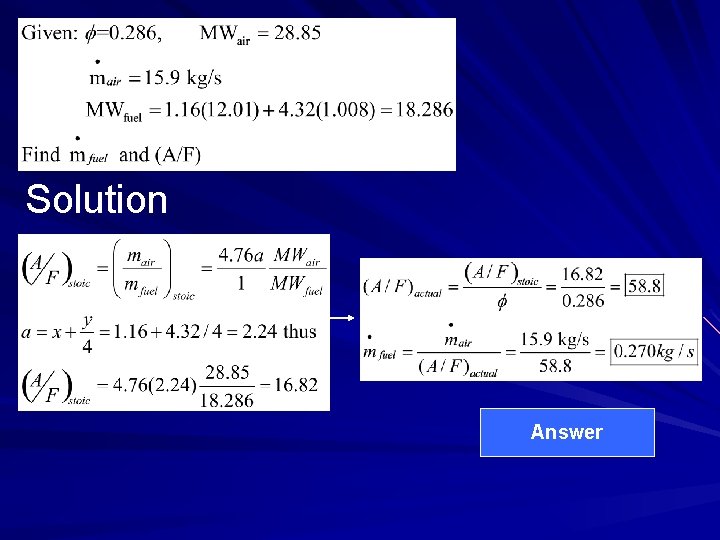
Solution Answer

Example 2 A natural gas-fired industrial boiler operates with an oxygen concentration of 3 mole percent in the flue gases. Determine the operating air-fuel ratio and equivalent ratio. Treat the natural gas as methane.

10 MW natural-gas burner air enter to the vertical pipes gas enter to the horizontal pipe on the left
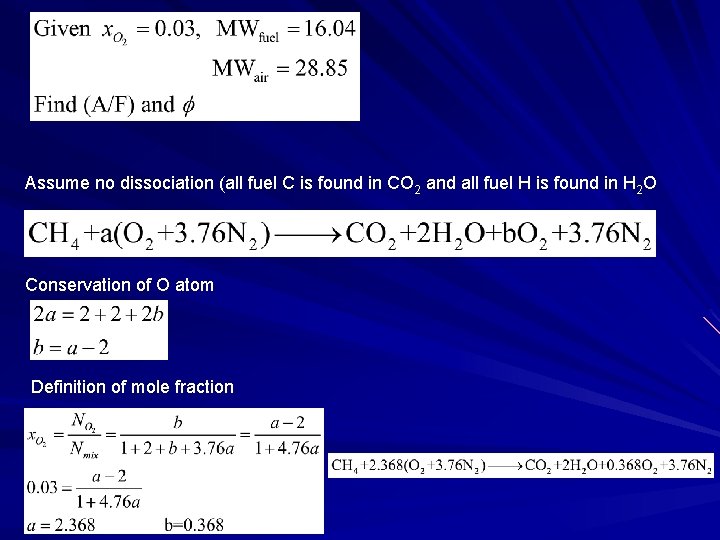
Assume no dissociation (all fuel C is found in CO 2 and all fuel H is found in H 2 O Conservation of O atom Definition of mole fraction Solution

To find Ф, we need to determine (A/F)stoic Answer
Example 3 Fuel ultimate analysis – Coal, as-fired: C 77%, H 2 5%, O 2 6%, S 1%, Ash 11% – Refuse: Ash 80%, Combustible 20% Flue gas analysis (by volume, dry basis) – CO 2 11. 90%, CO 0. 36%, O 2 7. 13%, N 2 80. 61% Determine the various product, the A: F ratio, and the excess air.

Solution 1. Find the carbon burned

Answer
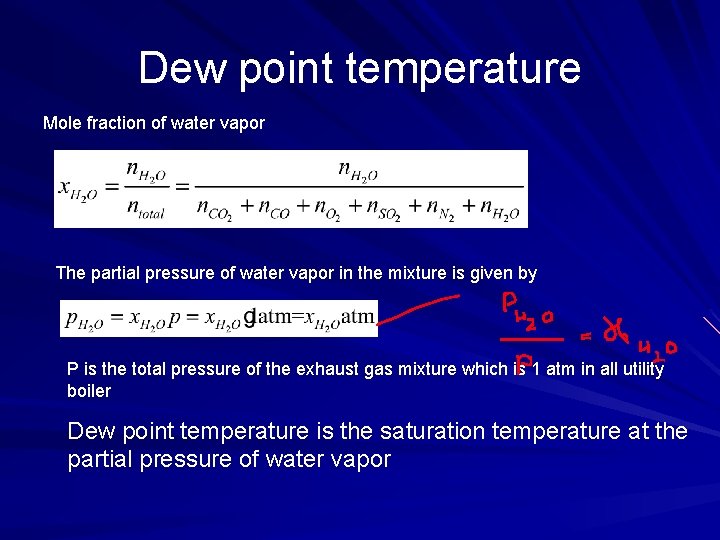
Dew point temperature Mole fraction of water vapor The partial pressure of water vapor in the mixture is given by P is the total pressure of the exhaust gas mixture which is 1 atm in all utility boiler Dew point temperature is the saturation temperature at the partial pressure of water vapor
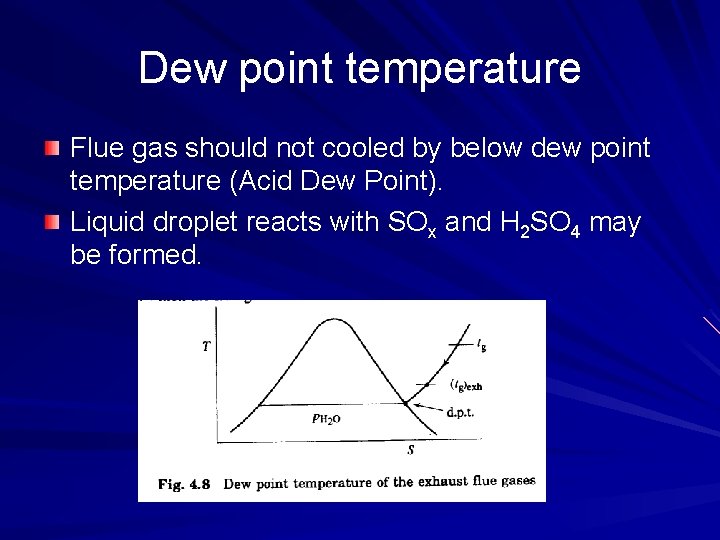
Dew point temperature Flue gas should not cooled by below dew point temperature (Acid Dew Point). Liquid droplet reacts with SOx and H 2 SO 4 may be formed.
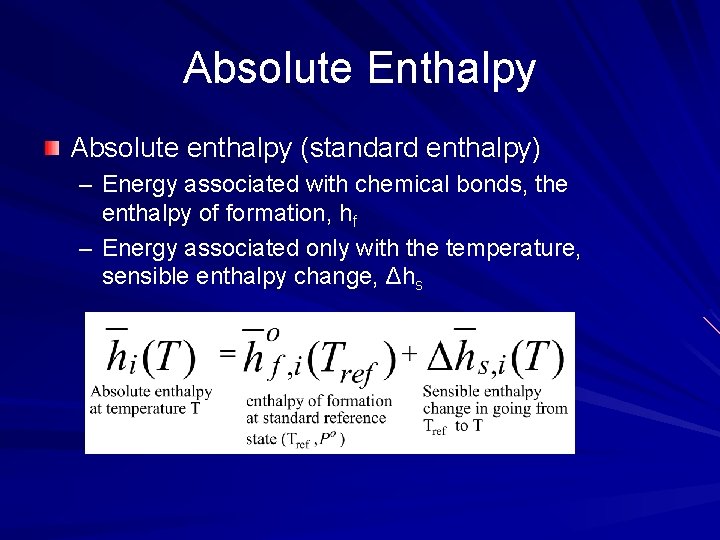
Absolute Enthalpy Absolute enthalpy (standard enthalpy) – Energy associated with chemical bonds, the enthalpy of formation, hf – Energy associated only with the temperature, sensible enthalpy change, Δhs
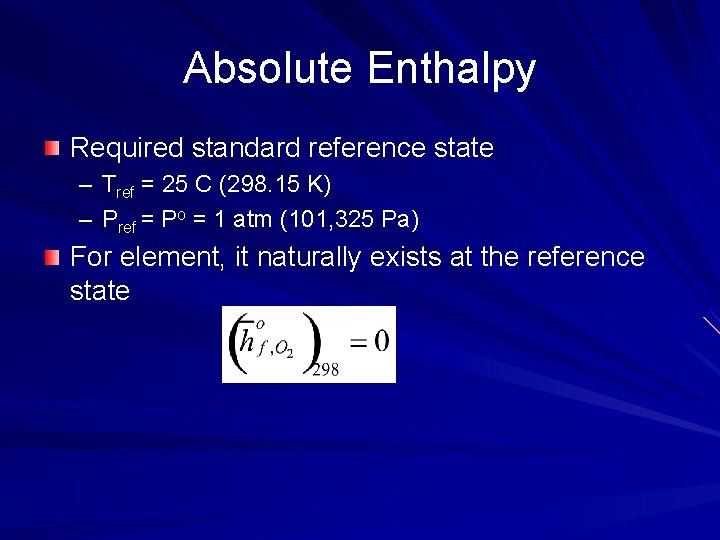
Absolute Enthalpy Required standard reference state – Tref = 25 C (298. 15 K) – Pref = Po = 1 atm (101, 325 Pa) For element, it naturally exists at the reference state
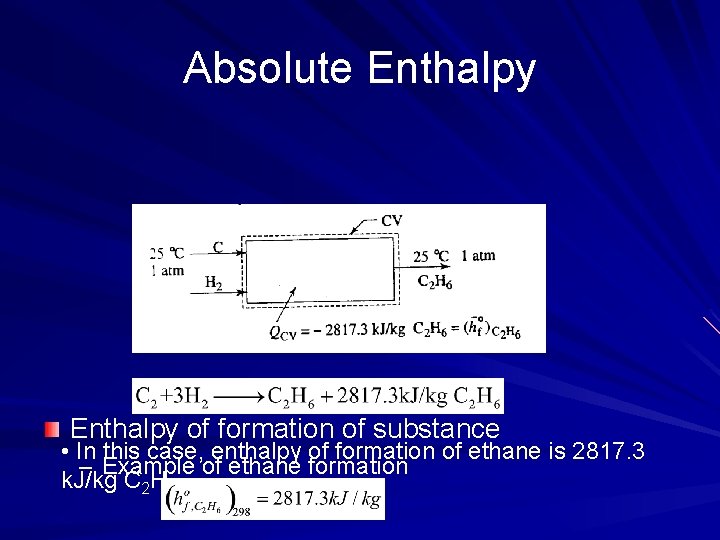
Absolute Enthalpy of formation of substance • In this case, enthalpy of formation of ethane is 2817. 3 – Example of ethane formation k. J/kg C 2 H 6
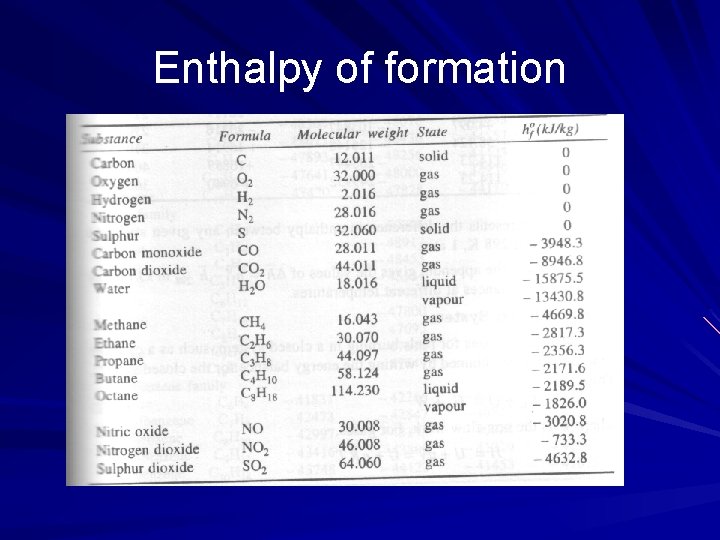
Enthalpy of formation
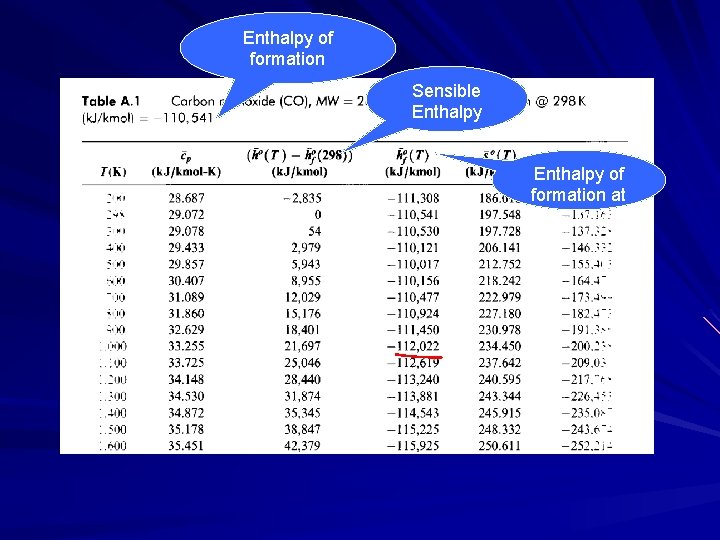
Enthalpy of formation Sensible Enthalpy of formation at T
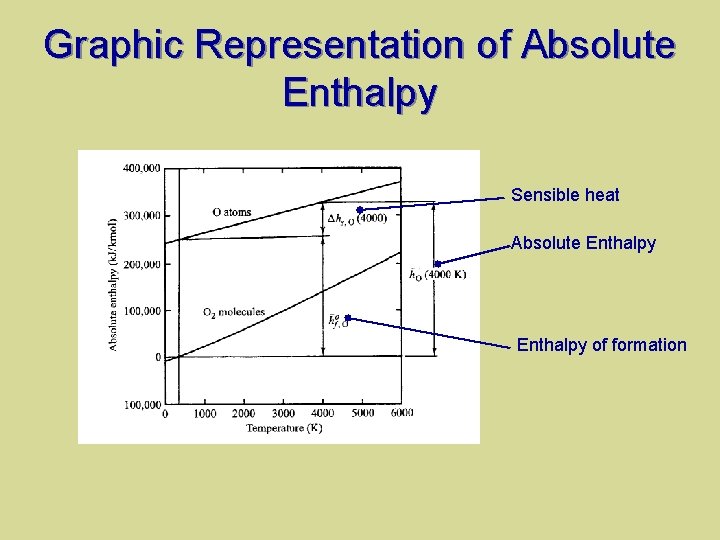
Graphic Representation of Absolute Enthalpy Sensible heat Absolute Enthalpy of formation
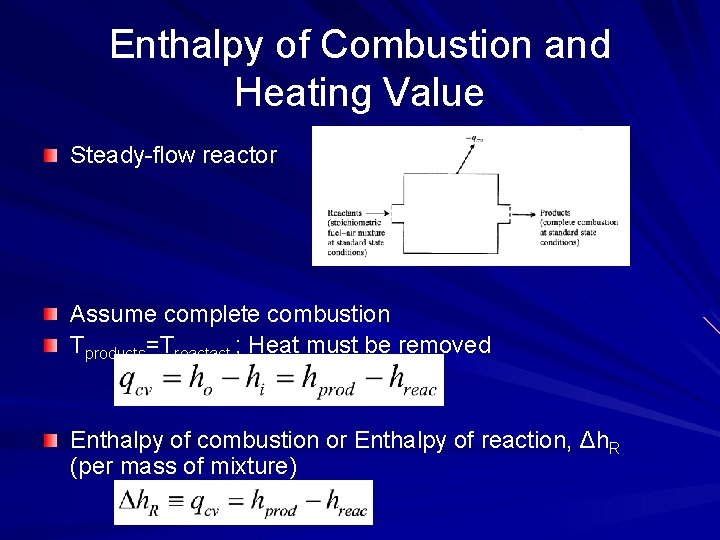
Enthalpy of Combustion and Heating Value Steady-flow reactor Assume complete combustion Tproducts=Treactact ; Heat must be removed Enthalpy of combustion or Enthalpy of reaction, Δh. R (per mass of mixture)
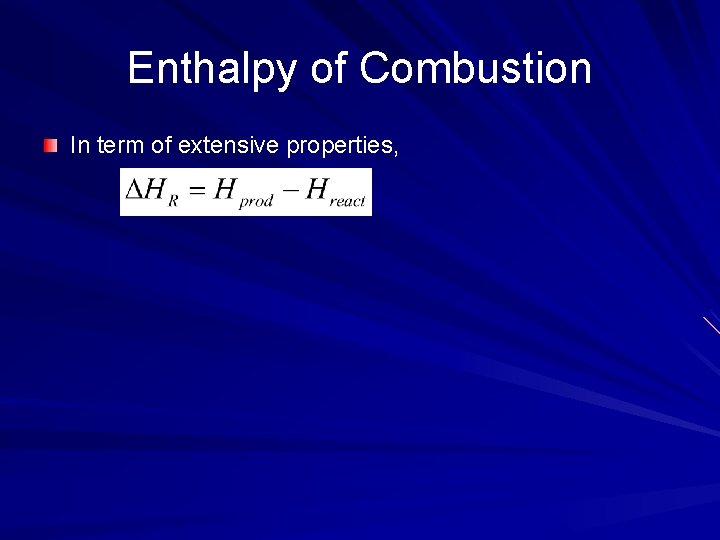
Enthalpy of Combustion In term of extensive properties,
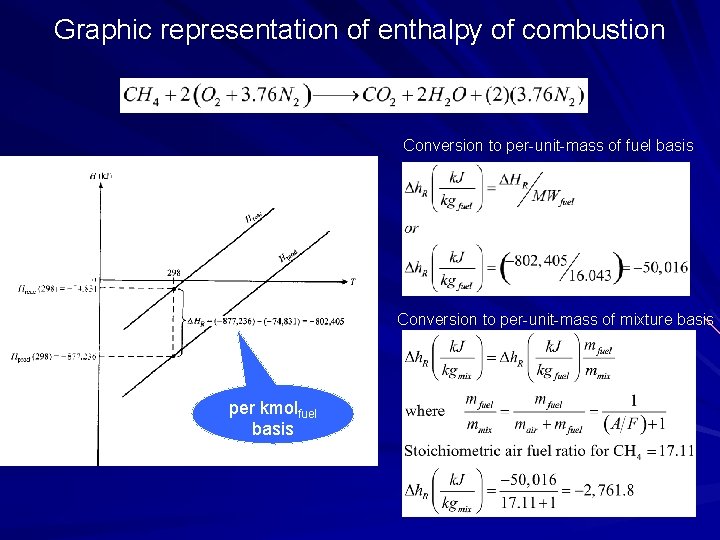
Graphic representation of enthalpy of combustion Conversion to per-unit-mass of fuel basis Conversion to per-unit-mass of mixture basis per kmolfuel basis
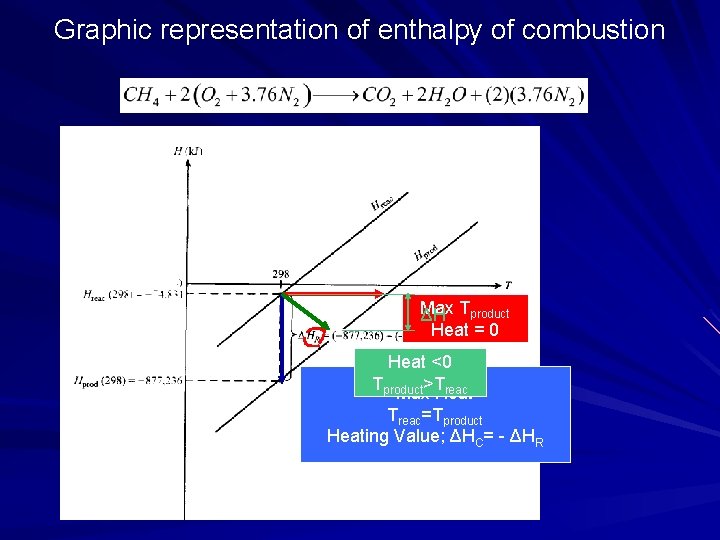
Graphic representation of enthalpy of combustion Max ΔH Tproduct Heat = 0 Heat <0 Tproduct>Treac Max Heat Treac=Tproduct Heating Value; ΔHC= - ΔHR

Heating Value Higher (upper) heating value, HHV – Water in products condenses to liquid Lower heating value, LHV – None of water is condensed
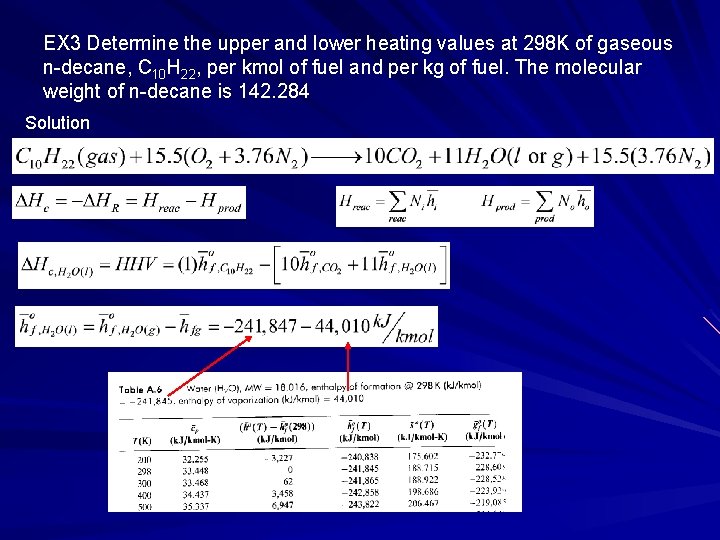
EX 3 Determine the upper and lower heating values at 298 K of gaseous n-decane, C 10 H 22, per kmol of fuel and per kg of fuel. The molecular weight of n-decane is 142. 284 Solution
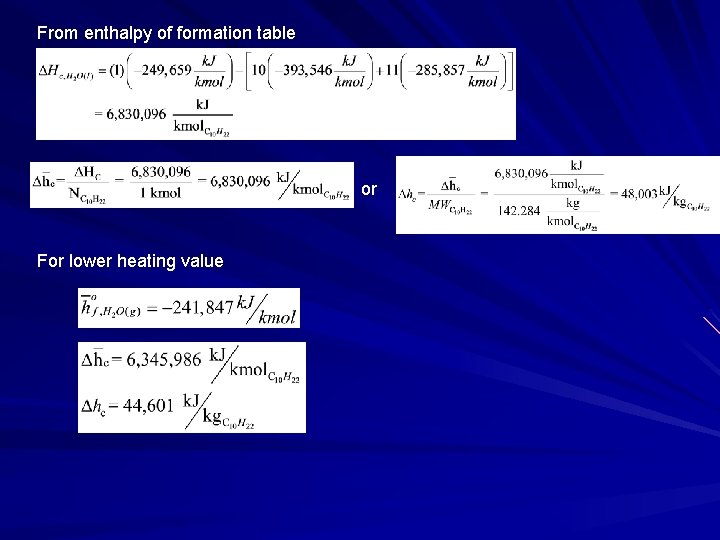
From enthalpy of formation table or For lower heating value

Graphic representation of this problem

Adiabatic Flame Temperature, Tad In power plant application, constant-pressure adiabatic flame temperature (Tad) is considered. No heat loss
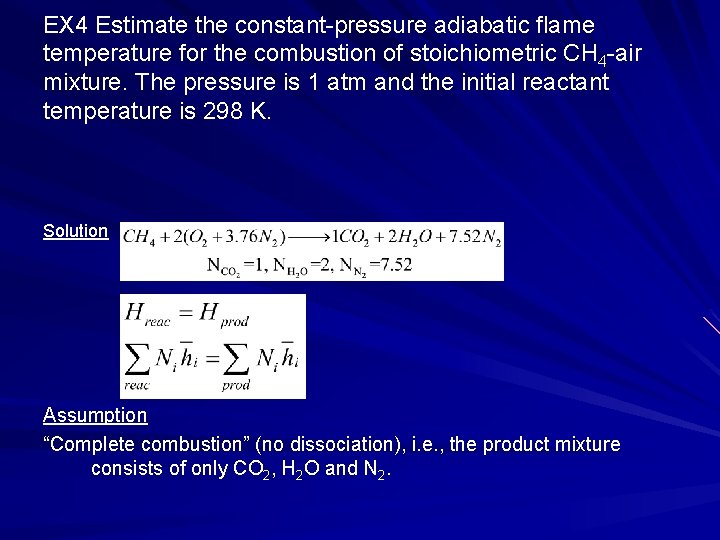
EX 4 Estimate the constant-pressure adiabatic flame temperature for the combustion of stoichiometric CH 4 -air mixture. The pressure is 1 atm and the initial reactant temperature is 298 K. Solution Assumption “Complete combustion” (no dissociation), i. e. , the product mixture consists of only CO 2, H 2 O and N 2.
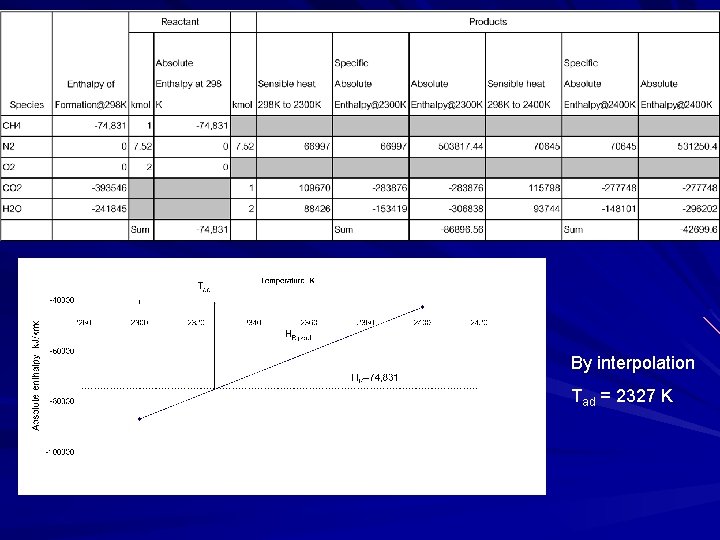
By interpolation Tad = 2327 K

Let’s think together; 1 From the adiabatic flame temperature calculation example (example 4), if the fuel -air mixture is more fuel-lean mixture and more fuel- rich, how will it influence on adiabatic flame temperature? Use theory, studied in this class, to discuss this question.

Let’s think together 2 If you are plant operator, you are asked to check the degree of rich-lean of the fuel-air mixture. Normally, the following parameters are measured at the plant. – Fuel flow rate – Flue gas composition Air flow rate and flue gas flow rate are normally not measured because the flow rate is very high. Is it possible to know that your fuel-air mixture is rich or lean? If it is possible, explain how to check it?

Let’s think together 3 In example 1, it was stated in the problem that the equivalent ratio of fuel-air mixture in gas turbine is 0. 286. In example 2, it was resulted from the calculation that the equivalent ratio of fuel-air mixture in boiler is 0. 84. Those figures is very close to the actual situation. Why the equivalent ratio of fuel-air mixture in gas turbine is very low. (too much excess air)?

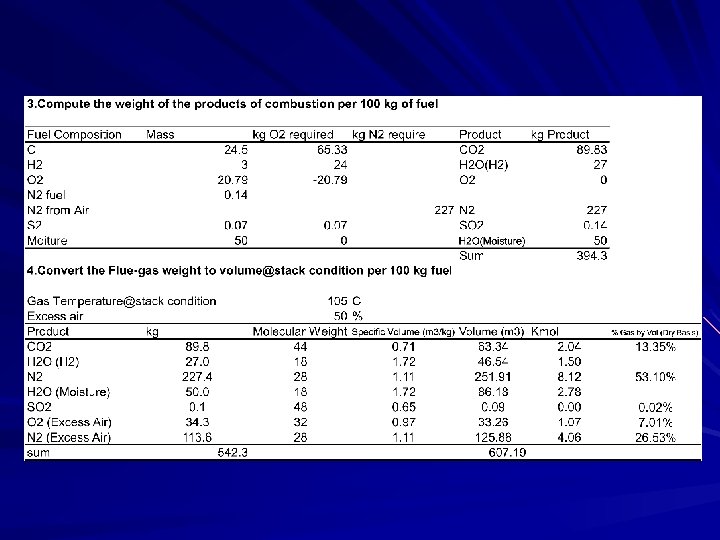
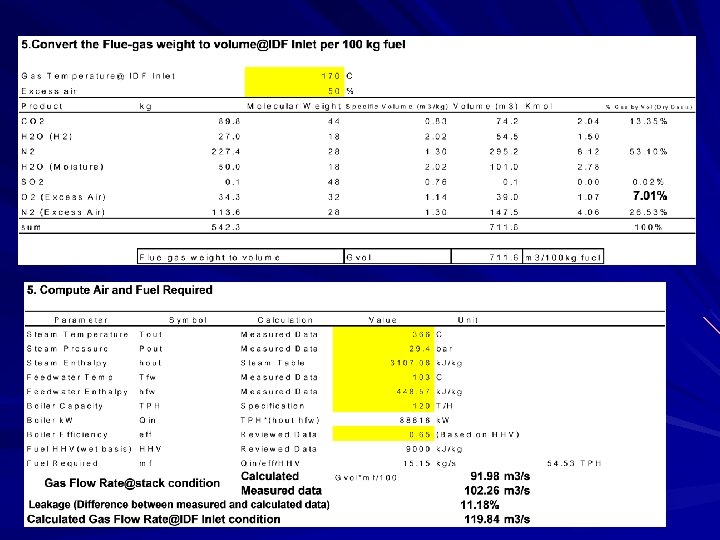
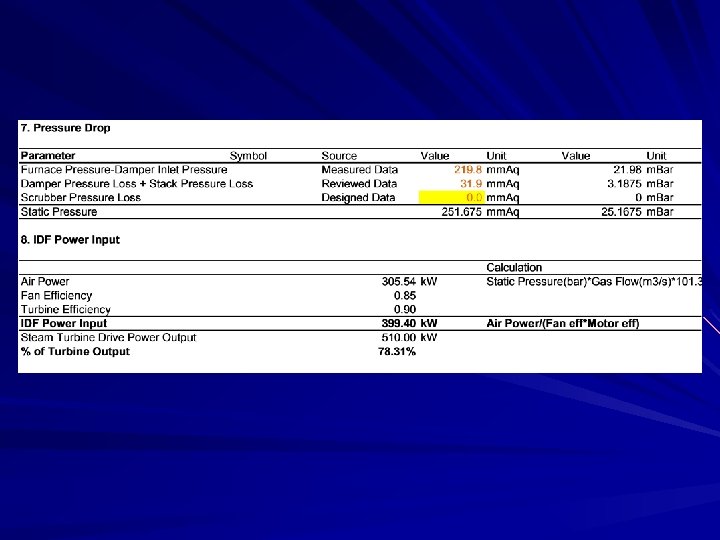
From my experience The power plant wanted to install the wet scrubber to the baggase-fired boiler. Mechanical engieer was asked to check whether the capacity of the induced-fan is big enough to work with more resistance from scrubber. To find the fan power, it is necessary to find out the gas flow rate though the fan. Please study how to work out with this problem
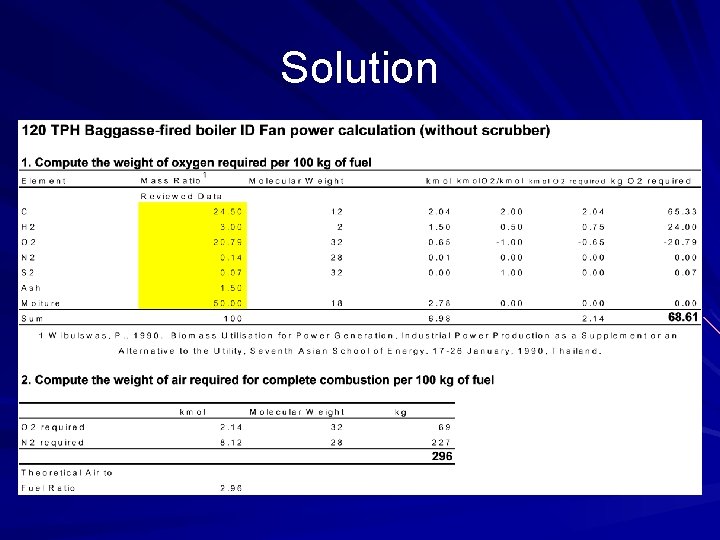
Solution
- Slides: 43