Combining ions Ionic bonding and dotcross diagrams 06022022
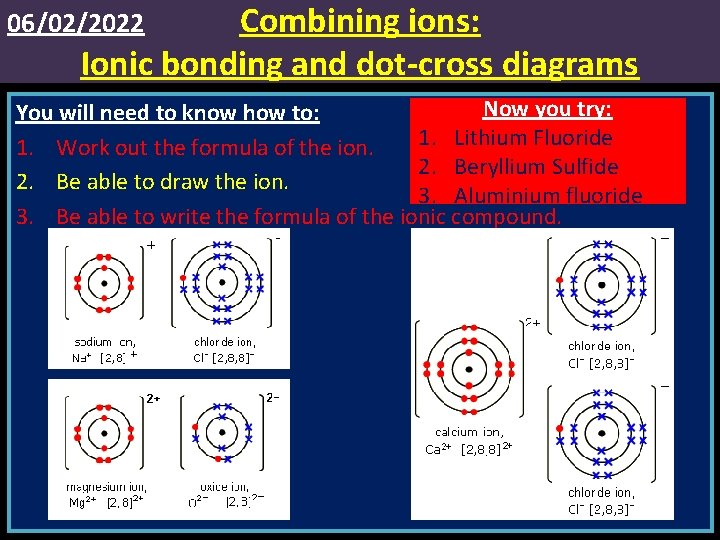
Combining ions: Ionic bonding and dot-cross diagrams 06/02/2022 Now you try: You will need to know how to: 1. Lithium Fluoride 1. Work out the formula of the ion. 2. Beryllium Sulfide 2. Be able to draw the ion. 3. Aluminium fluoride 3. Be able to write the formula of the ionic compound.
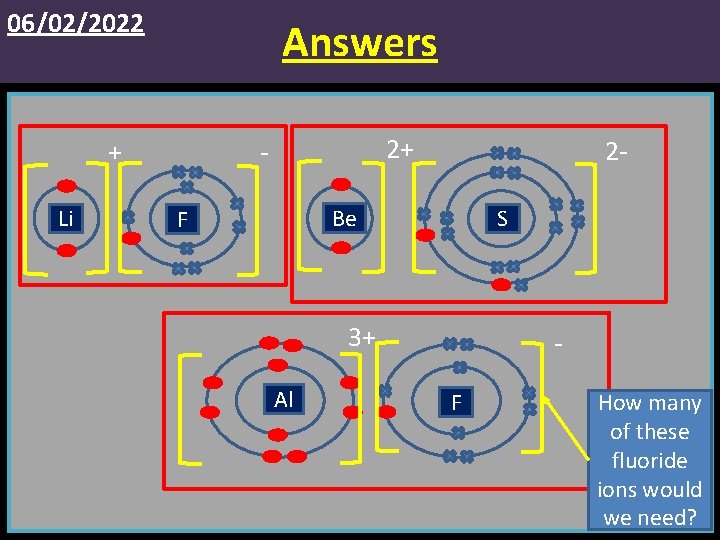
06/02/2022 Answers Li 2+ - + 2 - Be F S 3+ Al F How many of these fluoride ions would we need?

06/02/2022 Title: Properties of Ionic Compounds Complete the activities listed below Lesson aim: Know how ionic compounds form and the structure they take on. Explain the physical properties (e. g. melting point) of ionic compounds. 1. On your mini-whiteboard, sketch what you think ionic compounds look like: A. Label on any ions. B. Show any charges there might be. https: //www. youtube. com/watch? v=Ln. JTSSJ 6 Bqc Key words: Opposite charges, lattice, electrostatic, energy, free-flowing.

06/02/2022 (Giant) Ionic Lattice Ionic compounds are formed due to: Ø Electrostatic forces of attraction… Ø Between oppositely charged ions… Ø That arrange themselves in an alternating pattern: + - + -

06/02/2022 (Giant) Ionic Lattice § You will notice that each ion is surrounded by SEVERAL ions of the opposite charge. § Ionic compounds contains LOTS of ions… § Therefore lots of electrostatic forces of attraction holding together the ions… § Meaning that A LOT of energy is required to melt an ionic compound. § The ions are fixed in place.
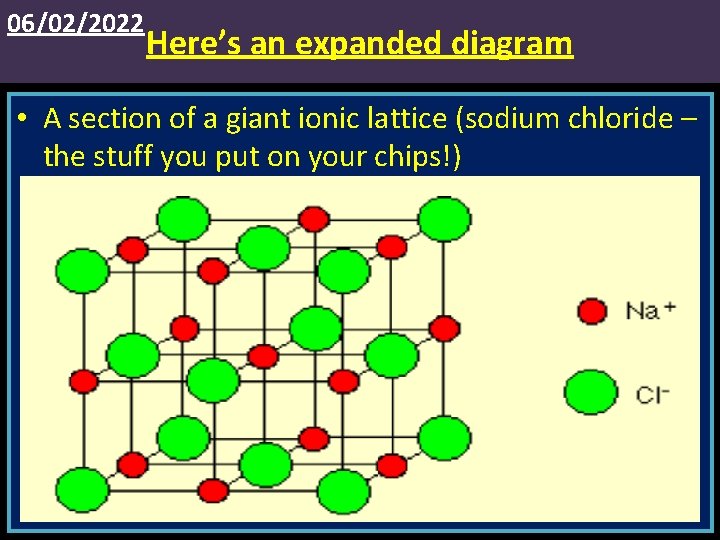
06/02/2022 Here’s an expanded diagram • A section of a giant ionic lattice (sodium chloride – the stuff you put on your chips!)
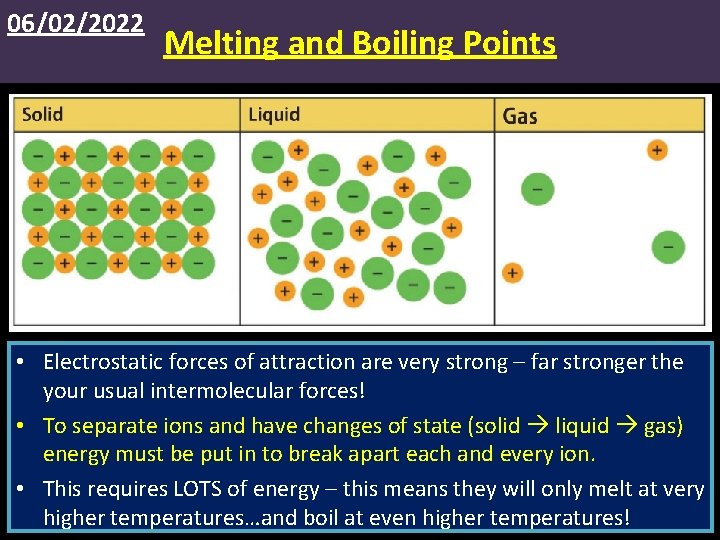
06/02/2022 Melting and Boiling Points • Electrostatic forces of attraction are very strong – far stronger the your usual intermolecular forces! • To separate ions and have changes of state (solid liquid gas) energy must be put in to break apart each and every ion. • This requires LOTS of energy – this means they will only melt at very higher temperatures…and boil at even higher temperatures!
06/02/2022 True or False Quiz: 1. Ionic compounds are made of alternating positive and negative ions. 2. Opposite charges repel each other. These are called electrostatic forces of repulsion. 3. Ions packed together in an ordered, regular structure is called a giant ionic lattice. 4. Electrostatic forces of attraction are very strong, stronger than intermolecular forces of attraction. 5. Ionic compounds have very low melting and boiling points.
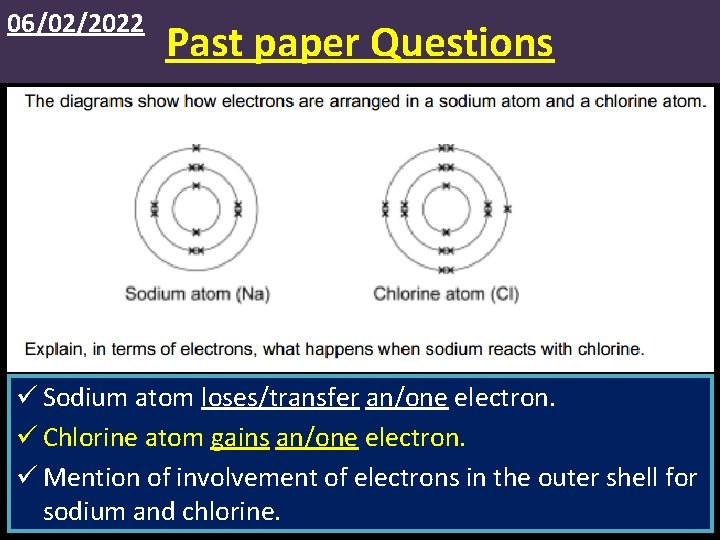
06/02/2022 Past paper Questions ü Sodium atom loses/transfer an/one electron. ü Chlorine atom gains an/one electron. ü Mention of involvement of electrons in the outer shell for sodium and chlorine.

06/02/2022 Past paper Questions

06/02/2022 Electrical Conductivity https: //www. youtube. com/watch? v=i. NN 0 ggzr. A 3 E • Watch the video about ionic compounds and electrical conductivity (We’ll play it twice). • Answer the questions on the sheet as we go along. 1. 2. 3. 4. 5. 6. Small electrical current. Dissolved salts. No, ionic substances do not conduct when solid. Molten (heated until liquid); dissolved in water (aqueous solution). Contains charged particles…that are free to move. In ionic solids the ions are held in fixed positions Cannot carry charge. Molten or dissolved: charged ions are free to move!

06/02/2022 Ionic or Not? https: //www. youtube. com/watch? v=4 Will. Wjx. RWw • From what you know you should be able to tell whether a substance is ionic or not by its electrical conductivity. • Identify, from the video, whether or not these are ionic substances: Ø Tap water: Ø Distilled water: Ø Salt water: Make predictions Ø Hydrochloric acid (HCl): first, then record Ø Sodium hydroxide (Na. OH): your answers on a Ø Sugar water: mini-whiteboard. Ø Vinegar (mostly CH 3 COOH): Ø Ethanol (CH 3 CH 2 OH): Ø Barium Sulfate (Ba. SO 4):

06/02/2022 Barium Sulfate: A surprising result? i. It is an ionic compound…(positive ion attracted to negative ion – held together by electrostatic forces of attraction) ii. It does not dissolve in water (is insoluble)… iii. There does not form a solution to conduct electricity… iv. Because its charged ions remain fixed and unable to carry charge.
- Slides: 13