Combinatorial chemistry Definition Combinatorial chemistry is a technique
Combinatorial chemistry § Definition üCombinatorial chemistry is a technique by which large numbers of different but structurally similar molecules are produced rapidly and submitted for pharmacological assay. üThis technique uses the same reaction conditions with the same reaction vessels to produce a large range of analogues. üTechnique invented in the late 1980 s and early 1990 s to enable applied to many molecules simultaneously. tasks to be § Combinatorial Library: üThe products produced from combinatorial synthesis are known as a combinatorial library. üLibraries may be a collection of individual compounds or mixtures of compounds. üScreening the components of a library for activity using high-throughput screening techniques enables the development team to select suitable compounds.
Application: Ø Applications of combinatorial chemistry are very wide. Scientists use combinatorial chemistry to create large population of molecules that can be screened efficiently. Ø B y producing larger, more diverse compound libraries, companies increase the probability that they will find novel compounds having significant therapeutic and commercial value. Ø Provides a stimulus for robot-controlled and immobilization strategies that allow high-throughput and multiple parallel approaches to drug discovery.

Advantages: v. Fast Combinatorial approach can give rise to million of compound in same time as it will take to produce one compound by traditional method of synthesis. v. Economical A negative result of mixture saves the effort of synthesis, purification & identification of each compound. v. Easy Isolation, purification & identification of active molecule from combinatorial library is relatively easy. v. Drug Discovery Mixed Combinatorial synthesis produces chemical pool. Probability of finding a molecule in a random screening process is proportional to the number of molecules subjected to the screening process. v. Drug Optimization Parallel synthesis produces analogues with slight differences which is required for lead optimization.

Disadvantages: ü Efficiency is highly affected by compound's size, solubility and function group. ü Compounds produced tend to be Achiral of Racemic.
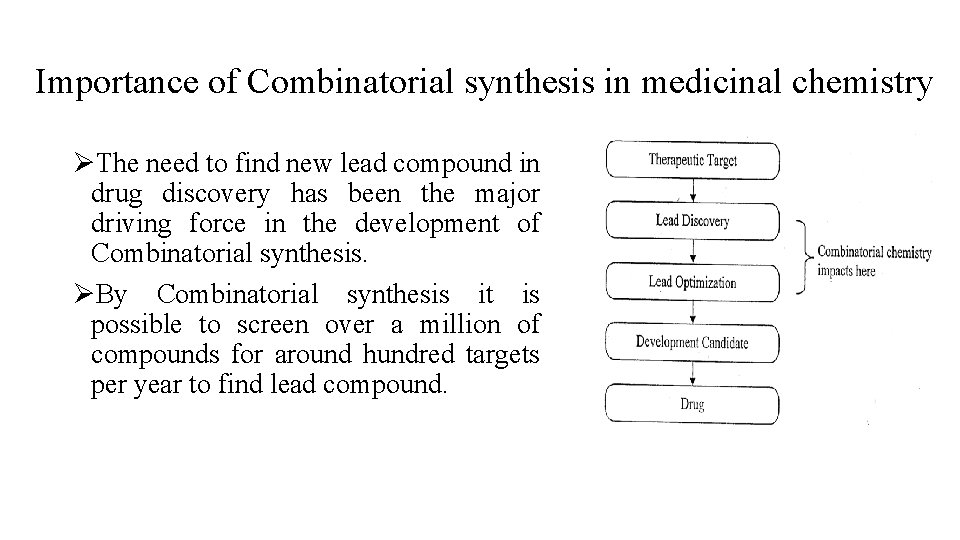
Importance of Combinatorial synthesis in medicinal chemistry ØThe need to find new lead compound in drug discovery has been the major driving force in the development of Combinatorial synthesis. ØBy Combinatorial synthesis it is possible to screen over a million of compounds for around hundred targets per year to find lead compound.
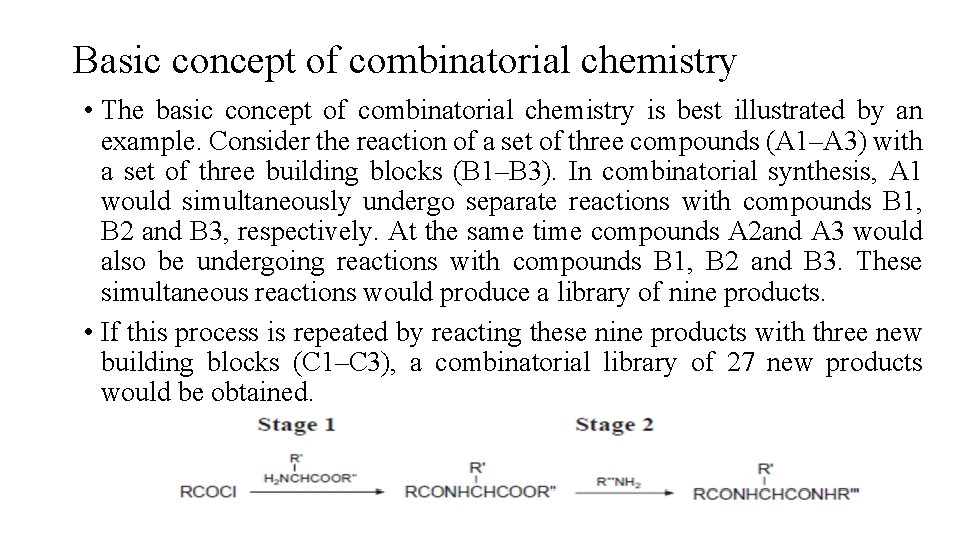
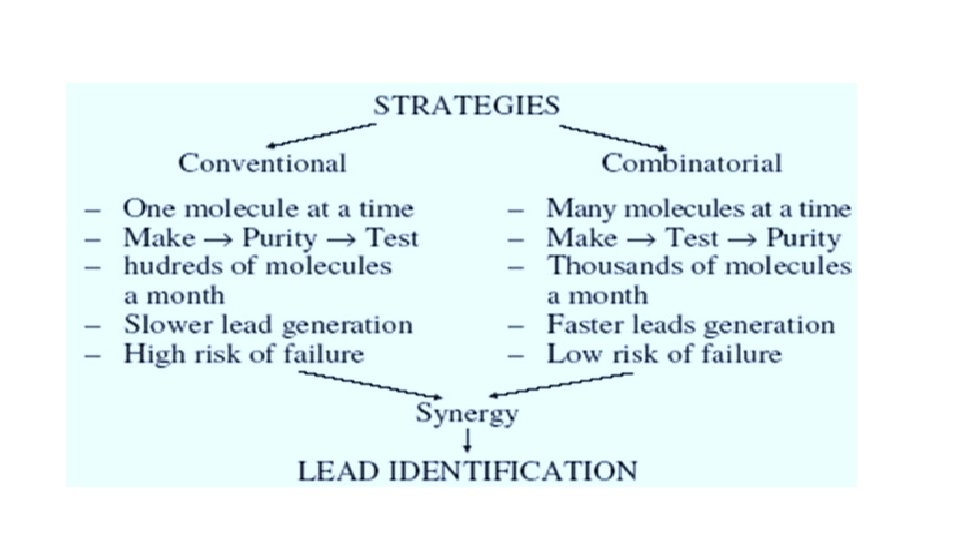
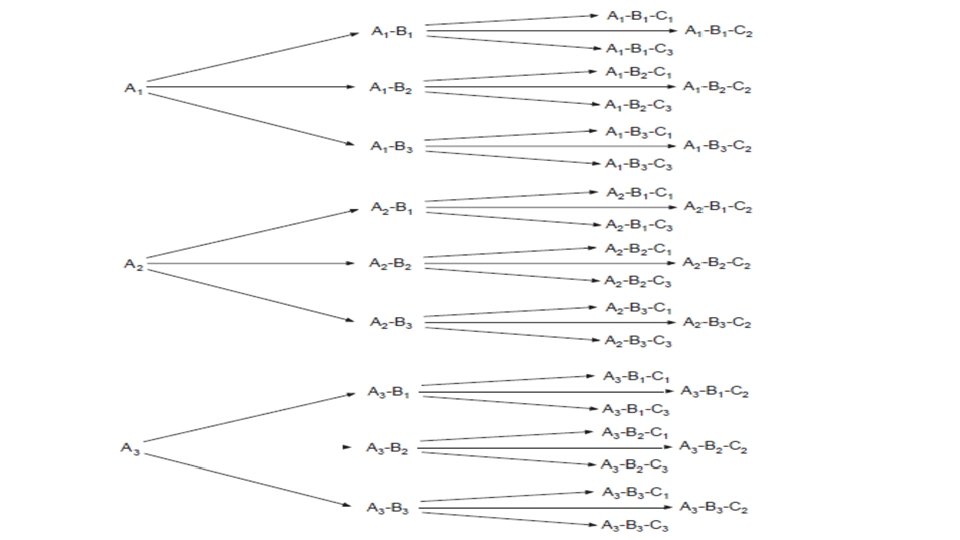
Basic concept of combinatorial chemistry • The basic concept of combinatorial chemistry is best illustrated by an example. Consider the reaction of a set of three compounds (A 1–A 3) with a set of three building blocks (B 1–B 3). In combinatorial synthesis, A 1 would simultaneously undergo separate reactions with compounds B 1, B 2 and B 3, respectively. At the same time compounds A 2 and A 3 would also be undergoing reactions with compounds B 1, B 2 and B 3. These simultaneous reactions would produce a library of nine products. • If this process is repeated by reacting these nine products with three new building blocks (C 1–C 3), a combinatorial library of 27 new products would be obtained.
Ideal criteria for combinatorial synthesis The reactions used when designing a combinatorial sequence should ideally satisfy the following criteria: 1. The reactions should be specific, relatively easy to carry out and give a high yield. 2. The reactions used in the sequence should allow for the formation of a wide range of structures for the final products as possible, including all the possible stereoisomers. 3. The reactions should be suitable for use in automated equipment. 4. The building blocks should be readily available. 5. The building blocks should be as diverse as possible so that the range of final products includes structures that utilize all types of bonding to bind to or react with the target. 6. It must be possible to accurately determine the structures of the final products.

The design of combinatorial syntheses • Two general strategies may be followed when designing a combinatorial synthesis. • Linear synthesis: In this case the building blocks are successively added to the preceding structure so that it grows in only one direction. • Template synthesis: This type of synthesis can proceed in different directions from an initial building block known as a template provided that the template has either the necessary functional groups or they can be generated during the course of the synthesis.
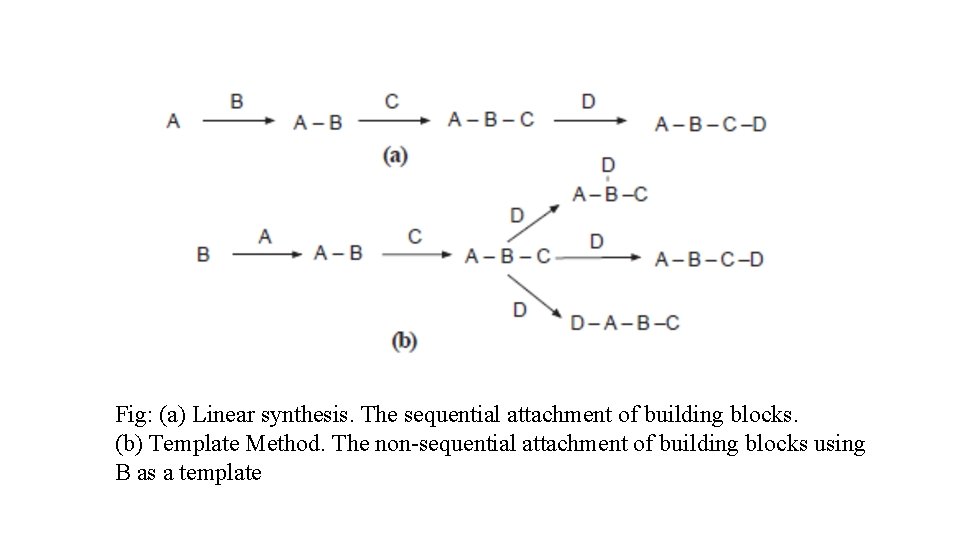
Fig: (a) Linear synthesis. The sequential attachment of building blocks. (b) Template Method. The non-sequential attachment of building blocks using B as a template
1. SOLID PHASE TECHNIQUES • Reactants are bound to a polymeric surface and modified whilst still attached. • Final product is released at the end of the synthesis. 1. 1 Advantages ü Specific reactants can be bound to specific beads. ü Beads can be mixed and reacted in the same reaction vessel. ü Products formed are distinctive for each bead and physically distinct. ü Excess reagents can be used to drive reactions to completion. ü Excess reagents and by products are easily removed.

ü Reaction intermediates are attached to bead and do not need to be isolated and purified. ü Automation is possible. ü Individual beads can be separated to isolate individual products. ü Polymeric support can be regenerated and re-used after cleaving the product. ü Beads can be mixed and reacted in the same reaction vessel. ü Products formed are distinctive for each bead and physically distinct. ü Individual beads can be separated to isolate individual products.
1. 2 Requirements ØA resin bead or a functionalised surface to act as a solid support. ØAn anchor or linker. ØA bond linking the compound to the linker. The bond must be stable to the reaction conditions used in the synthesis. ØA means of cleaving the product from the linker at the end. ØProtecting groups for functional groups not involved in the synthesis. 1. 3 Examples of Solid Supports üPartially cross-linked polystyrene beads. § Hydrophobic in nature. § Causes problems in peptide synthesis due to peptide folding. üSheppard’s polyamide resin - more polar. üTentagel resin - similar environment to ether or THF. üBeads, pins and functionalised glass surfaces.
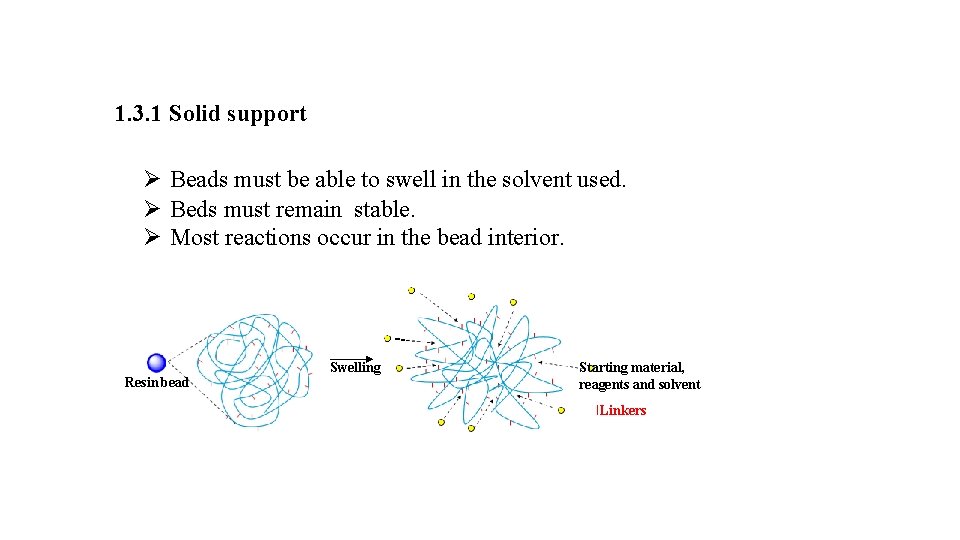
1. 3. 1 Solid support Ø Beads must be able to swell in the solvent used. Ø Beds must remain stable. Ø Most reactions occur in the bead interior. Resin bead Swelling Starting material, reagents and solvent Linkers
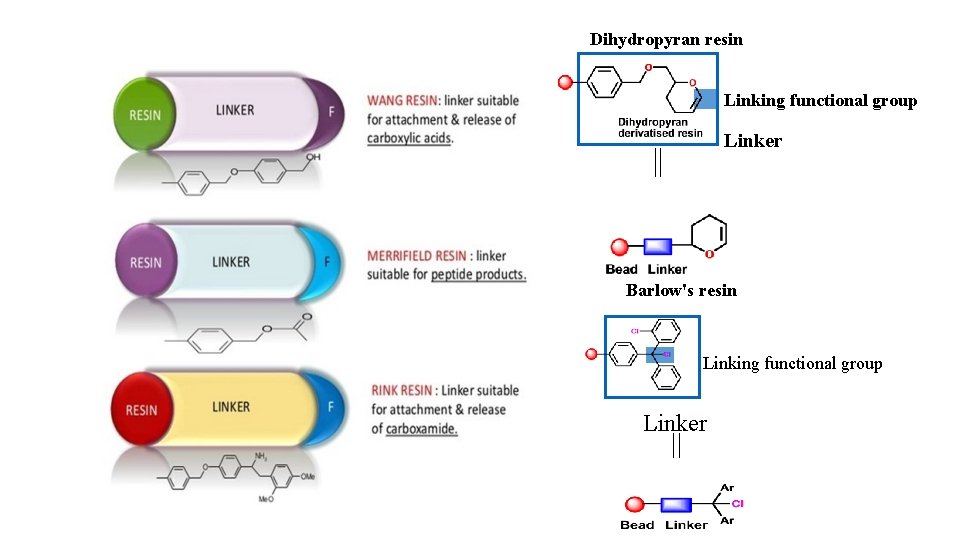
1. 4 Anchor or linker ü ü ü A molecular moiety which is covalently attached to the solid support, and which contains a reactive functional group. Allows attachment of the first reactant. The link must be stable to the reaction conditions in the synthesis but easily cleaved to release the final compound. Different linkers are available depending on the functional group to be attached and the desired functional group on the product. Resins are named to define the linker e. g. Merrifield, Wang, Rink
Dihydropyran resin Linking functional group Linker Barlow's resin Linking functional group Linker
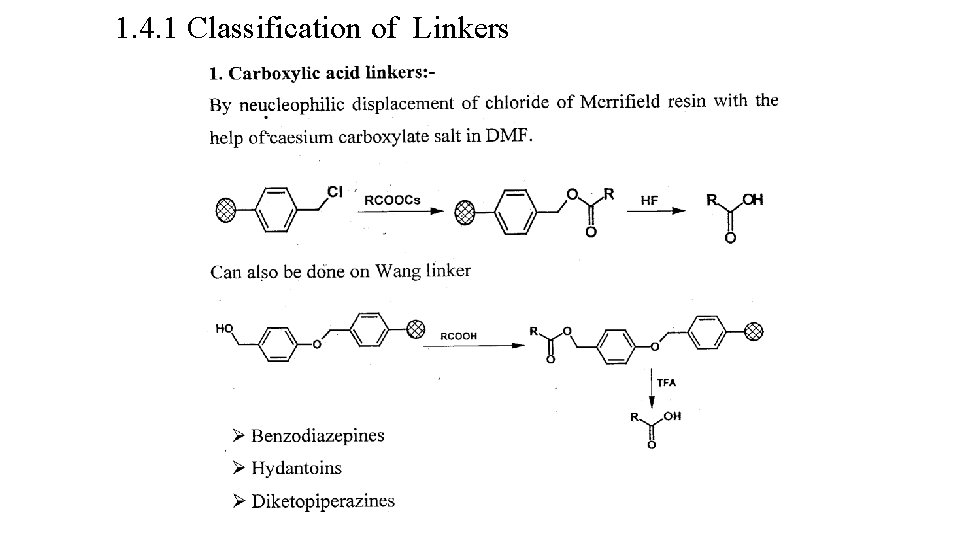
1. 4. 1 Classification of Linkers
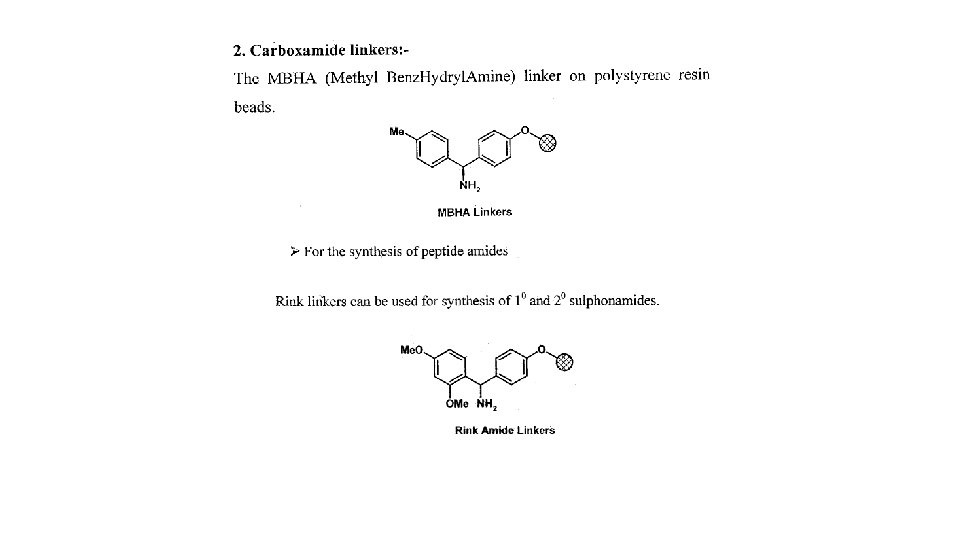

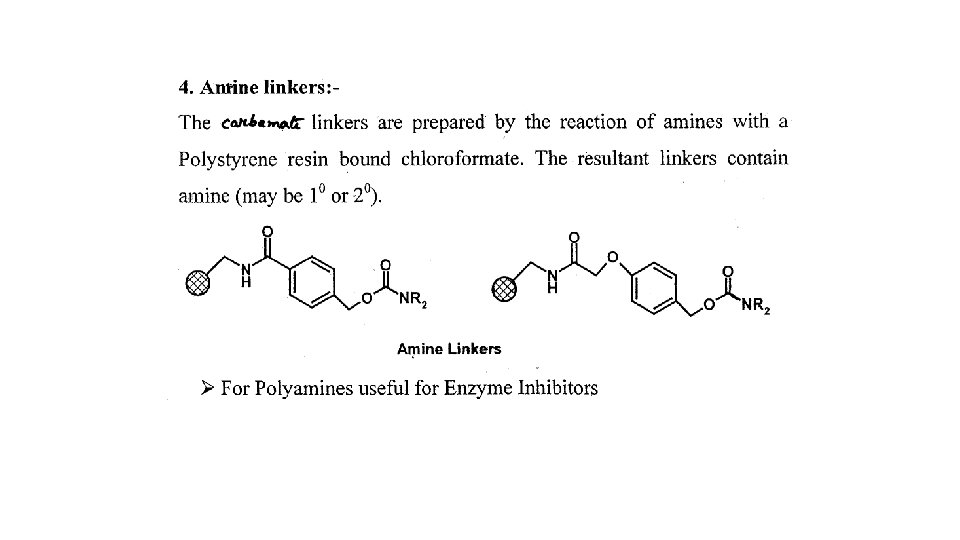
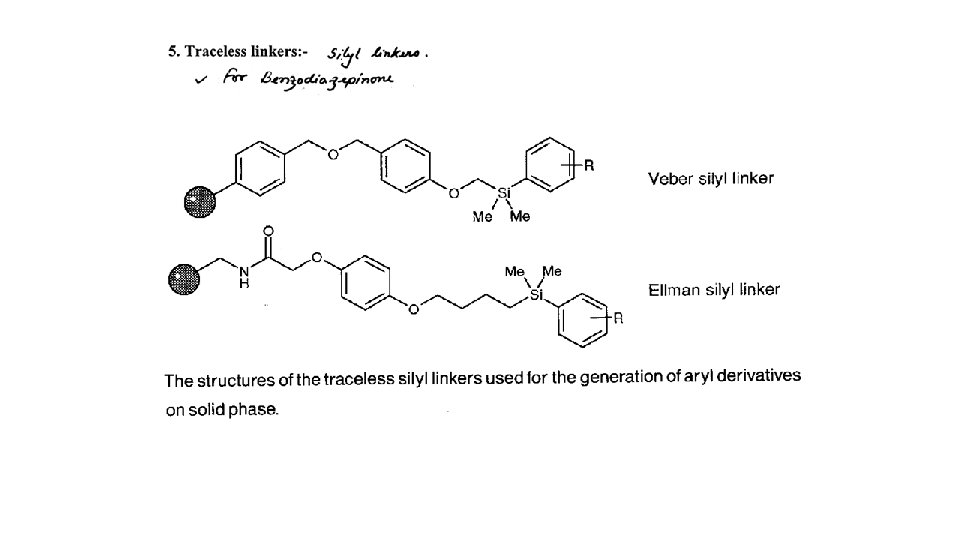


1. 5 Protecting agents and synthetic strategy 1. 5. 1 Protecting groups • Protect reactive functional groups that are not meant to react • Must be added easily in high yield under mild conditions • Must be stable under the reactions conditions used in the synthesis • Must be removed easily in high yield under mild conditions • Different protecting groups should be removed under different conditions
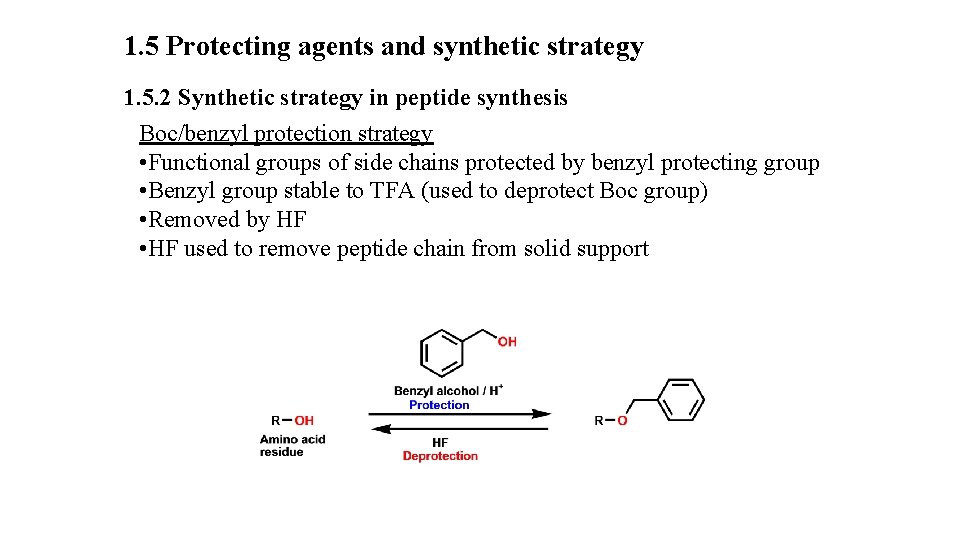
1. 5 Protecting agents and synthetic strategy 1. 5. 2 Synthetic strategy in peptide synthesis Boc/benzyl protection strategy • Functional groups of side chains protected by benzyl protecting group • Benzyl group stable to TFA (used to deprotect Boc group) • Removed by HF • HF used to remove peptide chain from solid support
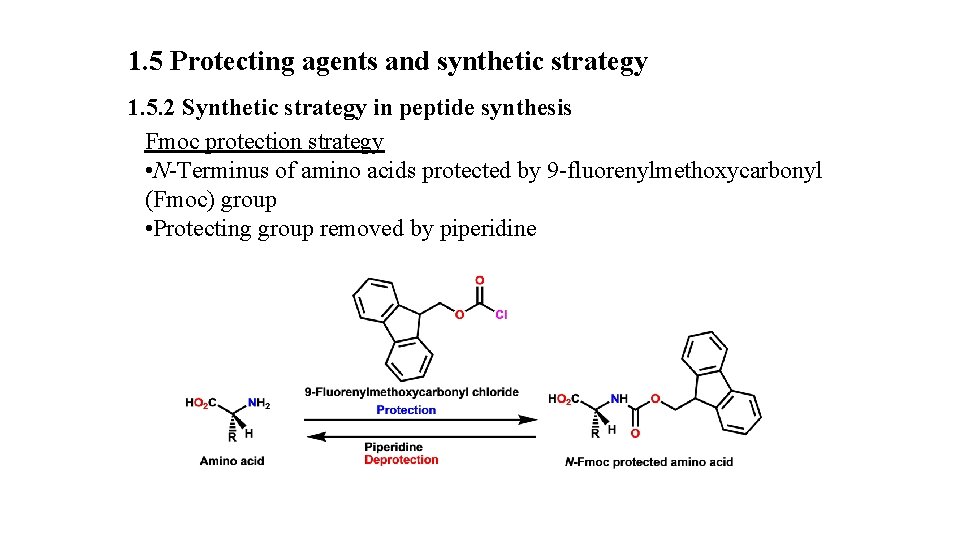
1. 5 Protecting agents and synthetic strategy 1. 5. 2 Synthetic strategy in peptide synthesis Fmoc protection strategy • N-Terminus of amino acids protected by 9 -fluorenylmethoxycarbonyl (Fmoc) group • Protecting group removed by piperidine
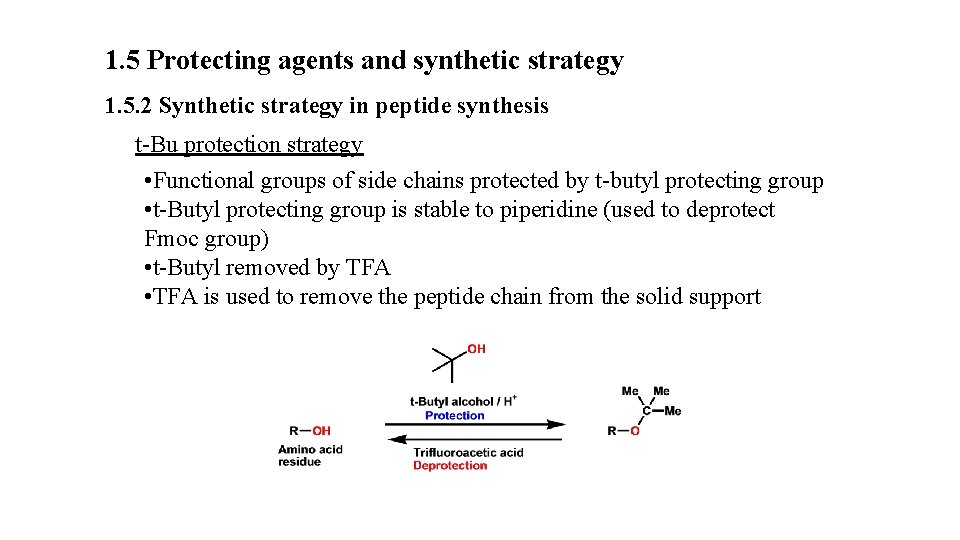
1. 5 Protecting agents and synthetic strategy 1. 5. 2 Synthetic strategy in peptide synthesis t-Bu protection strategy • Functional groups of side chains protected by t-butyl protecting group • t-Butyl protecting group is stable to piperidine (used to deprotect Fmoc group) • t-Butyl removed by TFA • TFA is used to remove the peptide chain from the solid support
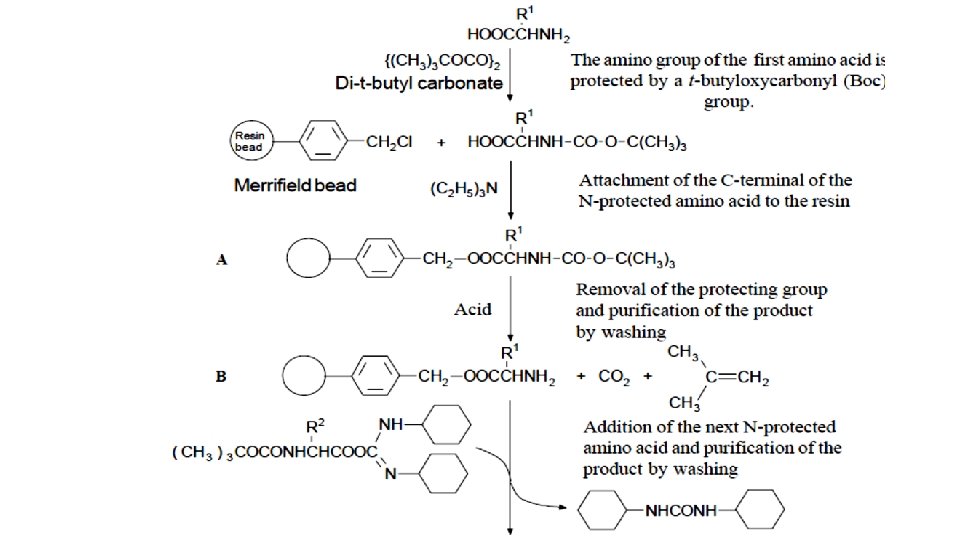
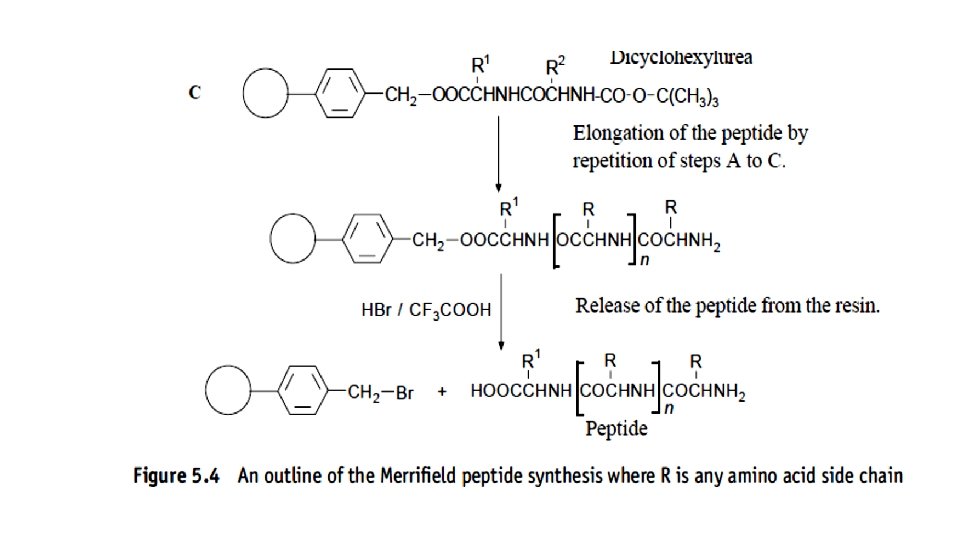
Merrifield peptide synthesis • The solid support method originated with the Merrifields (1963) solid support peptide synthesis. This method used polystyrene–divinylbenzene resin beads as a solid support for the product of each stage of the synthesis.

2. Parallel Synthesis 2. 1 Houghton’s Tea Bag Procedure • • Each tea bag contains beads and is labelled. Separate reactions are carried out on each tea bag. Combine tea bags for common reactions or work up procedures. A single product is synthesised within each tea bag. Different products are formed in different tea bags. Economy of effort - e. g. combining tea bags for workups. Cheap and possible for any lab. Manual procedure and is not suitable for producing large quantities of different products.
2. Parallel Synthesis Automated parallel synthesis Wells • Automated synthesisers are available with 42, 96 or 144 reaction vessels or wells. • Use beads or pins for solid phase support. • Reactions and work ups are carried out automatically. • Same synthetic route used for each vessel, but different reagents. • Different product obtained per vessel.
2. Parallel Synthesis Automated parallel synthesis of all 27 tri peptides from 3 amino acids ETC
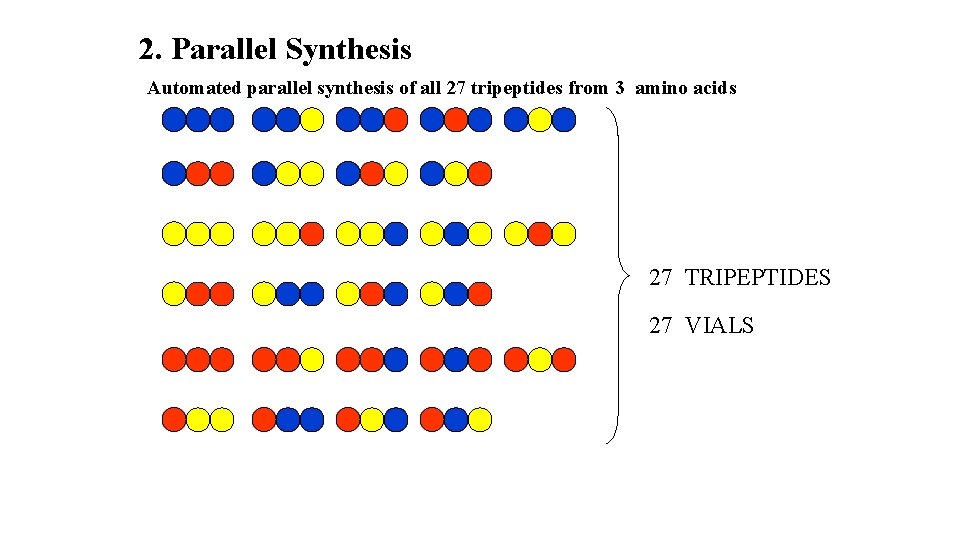
2. Parallel Synthesis Automated parallel synthesis of all 27 tripeptides from 3 amino acids 27 TRIPEPTIDES 27 VIALS

2. Parallel Synthesis 2. 2 Automated parallel synthesis AUTOMATED SYNTHETIC MACHINES

Parallel synthesis This technique is normally used to prepare combinatorial libraries that consist of separate compounds. In parallel synthesis the compounds are prepared in separate reaction vessels but at the same time, that is, in parallel. The array of individual reaction vessels often takes the form of either a grid of wells in a plastic plate or a grid of plastic rods called pins attached to a plastic base plate (Fig. 5. 7) that fits into a corresponding set of wells. In the former case the synthesis is carried out on beads placed in the wells whilst in the latter case it takes place on so-called plastic ‘crowns’ pushed on to the tops of the pins, the building blocks being attached to these crowns by linkers similar to those found on the resin beads. Both the well and pin arrays are used in the same general manner; the position of each synthetic pathway in the array and hence the structure of the product of that pathway is usually identified by a grid code.
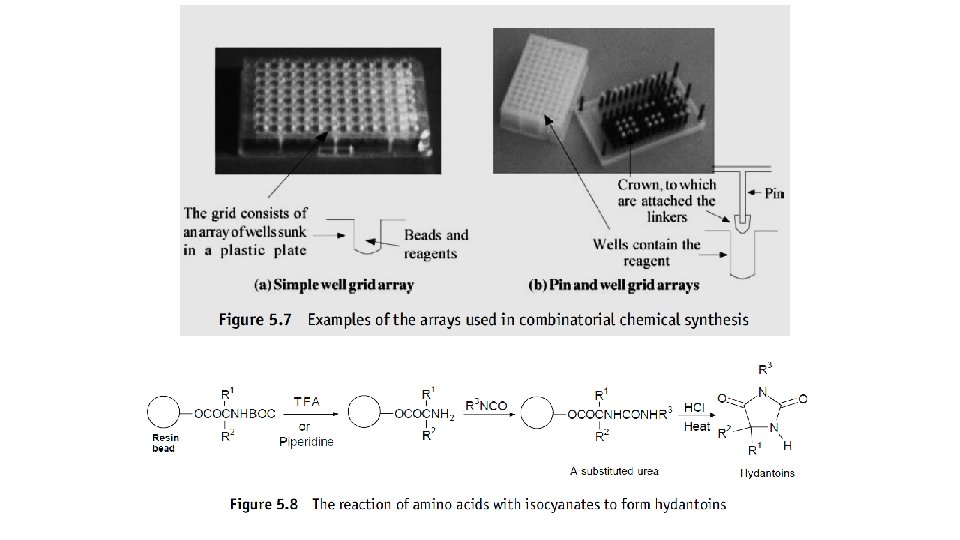
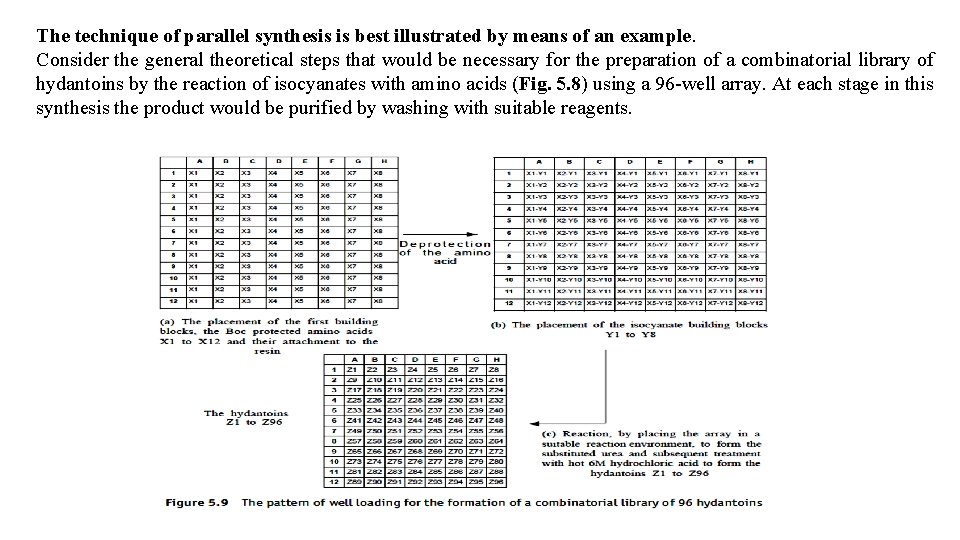
The technique of parallel synthesis is best illustrated by means of an example. Consider the general theoretical steps that would be necessary for the preparation of a combinatorial library of hydantoins by the reaction of isocyanates with amino acids (Fig. 5. 8) using a 96 -well array. At each stage in this synthesis the product would be purified by washing with suitable reagents.
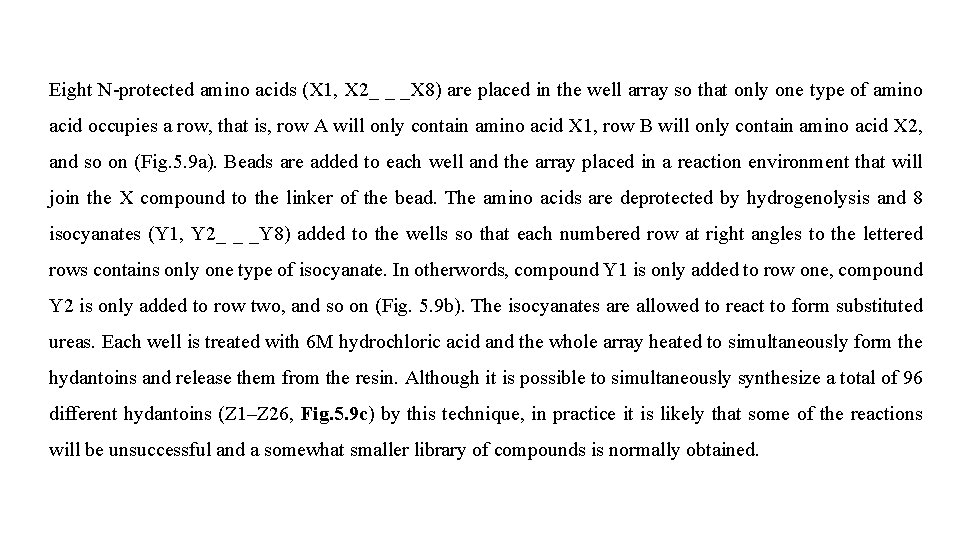
Eight N-protected amino acids (X 1, X 2_ _ _X 8) are placed in the well array so that only one type of amino acid occupies a row, that is, row A will only contain amino acid X 1, row B will only contain amino acid X 2, and so on (Fig. 5. 9 a). Beads are added to each well and the array placed in a reaction environment that will join the X compound to the linker of the bead. The amino acids are deprotected by hydrogenolysis and 8 isocyanates (Y 1, Y 2_ _ _Y 8) added to the wells so that each numbered row at right angles to the lettered rows contains only one type of isocyanate. In otherwords, compound Y 1 is only added to row one, compound Y 2 is only added to row two, and so on (Fig. 5. 9 b). The isocyanates are allowed to react to form substituted ureas. Each well is treated with 6 M hydrochloric acid and the whole array heated to simultaneously form the hydantoins and release them from the resin. Although it is possible to simultaneously synthesize a total of 96 different hydantoins (Z 1–Z 26, Fig. 5. 9 c) by this technique, in practice it is likely that some of the reactions will be unsuccessful and a somewhat smaller library of compounds is normally obtained.
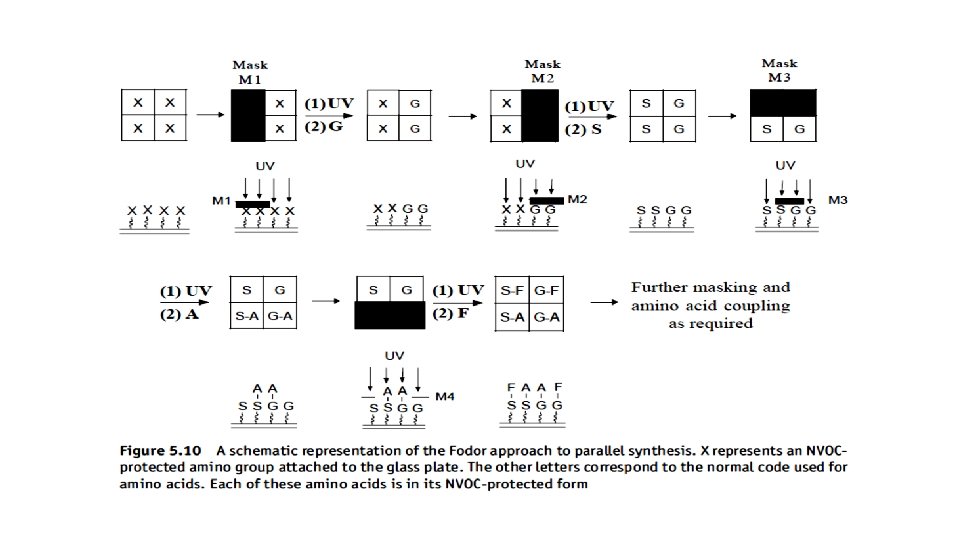
A photolithography mask (M 1) is placed over the plate so that only a specific area of the plate can be irradiated with UV light (Fig. 5. 10). This results in removal of the NVOC protecting group from the amino groups in the irradiated area. The entire plate is exposed to the first activated NVOC-protected amino acid. However, it will only bond to the amino groups exposed in the irradiated area (Step A). The process is repeated using a new mask (M 2) and a second activated NVOC-protected amino acid attached to the exposed amino groups (Step B). This process is repeated using different masks (M 3, etc. ) until the desired library is obtained, the structure of the peptide occupying a point on the plate depending on the masks used and the activated NVOC-protected amino acid used at each stage in the synthesis. The technique is so precise that it has been reported that each compound occupies an area of about 50 m × 50 m. A record of the way in which the masks are used will determine both the order in which the amino acids are added and, as a result, the structures of each of the peptides at specific coordinates on the plate.
3. Mixed Combinatorial Synthesis Aims • To use a standard synthetic route to produce a large variety of different analogues where each reaction vessel or tube contains a mixture of products • The identities of the structures in each vessel are not known with certainty • Useful for finding a lead compound • Capable of synthesising large numbers of compounds quickly • Each mixture is tested for activity as the mixture • Inactive mixtures are stored in combinatorial libraries • Active mixtures are studied further to identify active component
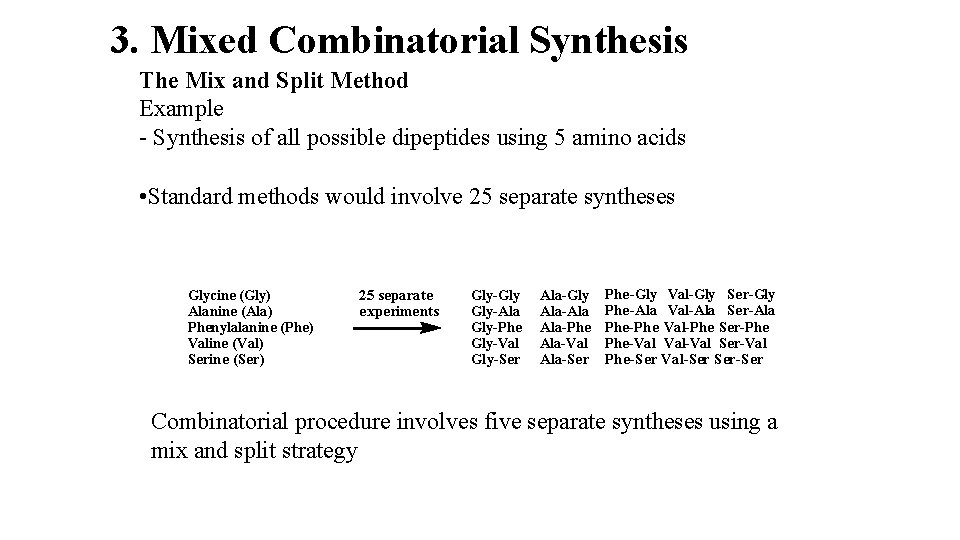
3. Mixed Combinatorial Synthesis The Mix and Split Method Example - Synthesis of all possible dipeptides using 5 amino acids • Standard methods would involve 25 separate syntheses Glycine (Gly) Alanine (Ala) Phenylalanine (Phe) Valine (Val) Serine (Ser) 25 separate experiments Gly-Gly Gly-Ala Gly-Phe Gly-Val Gly-Ser Ala-Gly Ala-Ala Ala-Phe Ala-Val Ala-Ser Phe-Gly Val-Gly Ser-Gly Phe-Ala Val-Ala Ser-Ala Phe-Phe Val-Phe Ser-Phe Phe-Val Val-Val Ser-Val Phe-Ser Val-Ser Ser-Ser Combinatorial procedure involves five separate syntheses using a mix and split strategy
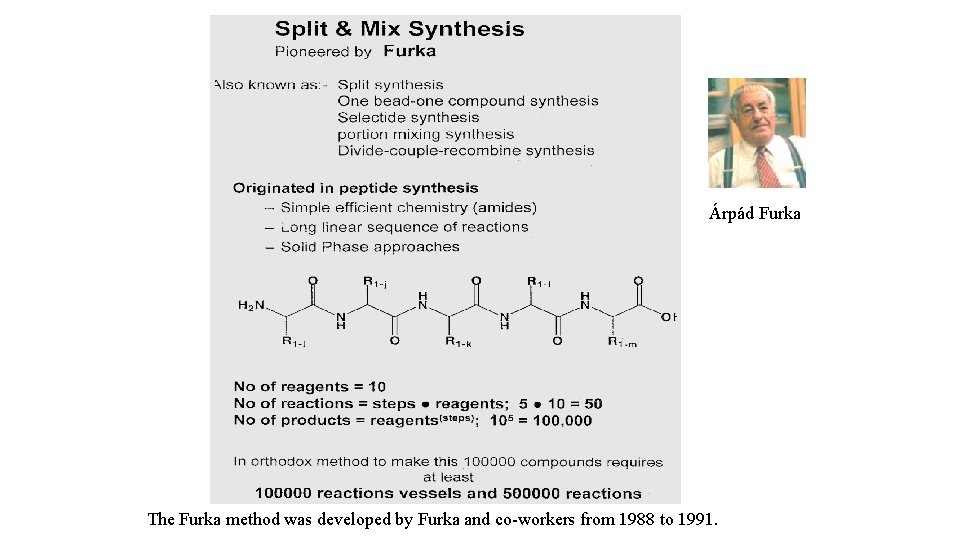
Árpád Furka The Furka method was developed by Furka and co-workers from 1988 to 1991.
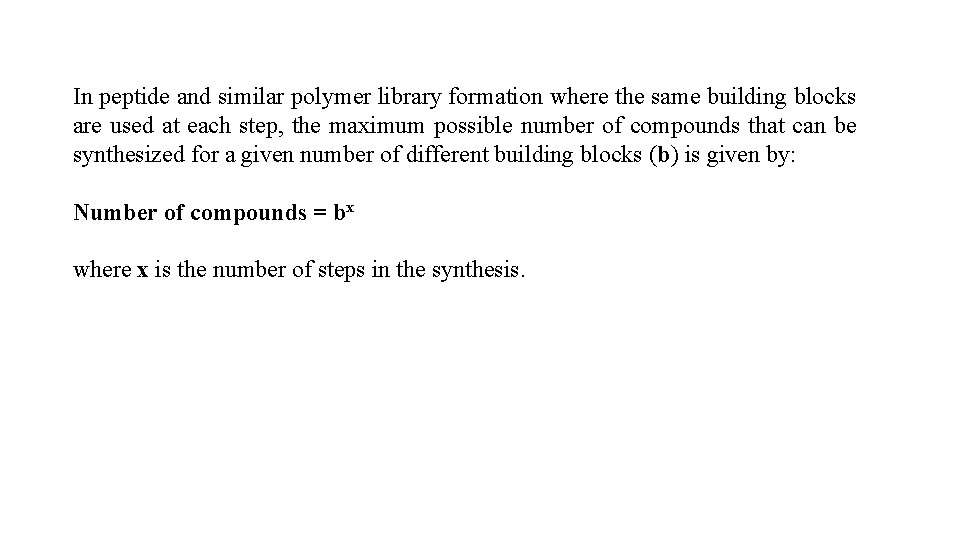
In peptide and similar polymer library formation where the same building blocks are used at each step, the maximum possible number of compounds that can be synthesized for a given number of different building blocks (b) is given by: Number of compounds = bx where x is the number of steps in the synthesis.

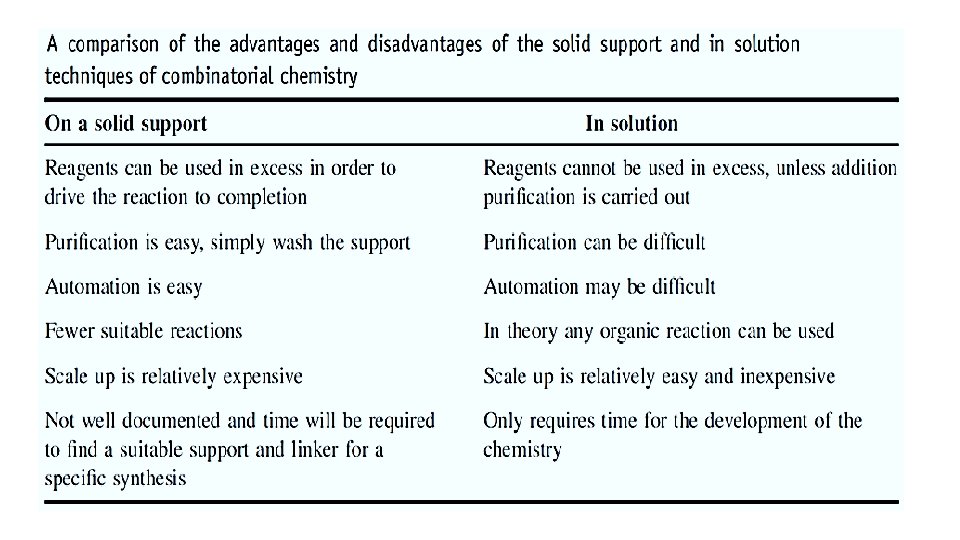
Solution Phase Technique ØWhen combinatorial chemistry first emerged, the initial focus was on solid-phase approaches due to the many advantages. ØSolution chemistry was not regarded as being suitable for combinatorial chemistry because of the often tedious isolation and purification. ØIt was first used for easily synthesized compound classes [amides, sulfonamides, urease, heterocyclic (thiazide)]. ØPresently, solution-phase combinatorial synthesis is attracting more interest because of some advantages.

Advantages ØMany more reactions are optimized in solution-phase. ØAll reactive groups of the starting materials are available. ØNo limitations of thermal or chemical stability of the resin. ØSynthesis is shorter by one or two steps. ØReactions in solution often need considerably less time. ØReactions that involve insoluble components are confined to solution phase. ØReactions can be followed conveniently by simple means (TLC, NMR, UV). ØIn general, the reaction volumes in relation to the amount of product are smaller.
- Slides: 50