COLUMN PACKING TECHNIQUE FOR HPLC COLUMN CONTENTS Introduction
















- Slides: 26

COLUMN PACKING TECHNIQUE FOR HPLC COLUMN CONTENTS Introduction Principle Practical consideration in column chromatography Column packing technique Dry packing method Wet or slurry method Solvent use Factors affecting to column efficiency Application 1

INTRODUCTION • Chromatography is defined as a process by which solutes are separated by a differential migration process in a system consisting of two or more phases one of which moves continuously in a given direction and in which the individual substances exhibit different mobilities by reason of differences in adsorption, partition, solubility, vapour pressure, molecular size, or ionic charge density. 2

• Reverse Phase Chromatography The term “Reverse Phase Chromatography” was used because RP is a form of partition chromatography where chemically bonded phase is hydrophobic or non-polar (e. g. octadecyl group), and the starting mobile phase (e. g. water) must be more polar than the stationary phase. normal phase chromatograph where the stationary phase is polar or hydrophilic and the starting mobile phase is more non-polar or hydrophobic than the stationary phase 3

PRINCIPLE • HPLC adsorption chromatography: 1) It based on liquid solid adsorption in which s. p solid. In such packing solute completes with the eluting solvent for size of the solid; retention result of absorption. 2) It is known that the rate of adsorption varies with a given adsorbent for different material. This principle of selective adsorption is used in column chromatography HPLC partition chromatography Most widely used solid support is silica gel. this material adsorb water strongly M. P polarity different. RP Chromatography are used. 4

Practical Consideration in column chromatography Chromatographic column Adsorbents Preparation of adsorbents Developers Solvents Packing of column Sample application Elution procedure Detectors 5

Types of column G. C 1)Packed column 2)Capillary Column(1 mm internal diameter) • Capillary column Main 2 types • Packed column with solid particale • Open tubular Column open tubular column two types 1) wall coated Open tubular Column(WCOT) 2)support-coated Open tubular Column(SCOT) 3)porous layer Open tubular Column(PLOT) • Column made of heavy glass or stainless steel tubing to withstand in high pressure. • It must effectively run in high temp. 6

Types of column HPLC Standard column Radial compression Narrow bore Length 10 To 30 cm 10 cm long 10 To 30 cm Internal diameter 4 to 5 mm 8 mm bore 1 to 2 mm bore Particle size 3 to 5 μm upto 10 μm 3 to 5 μm 7

Chromatographic column: - 8

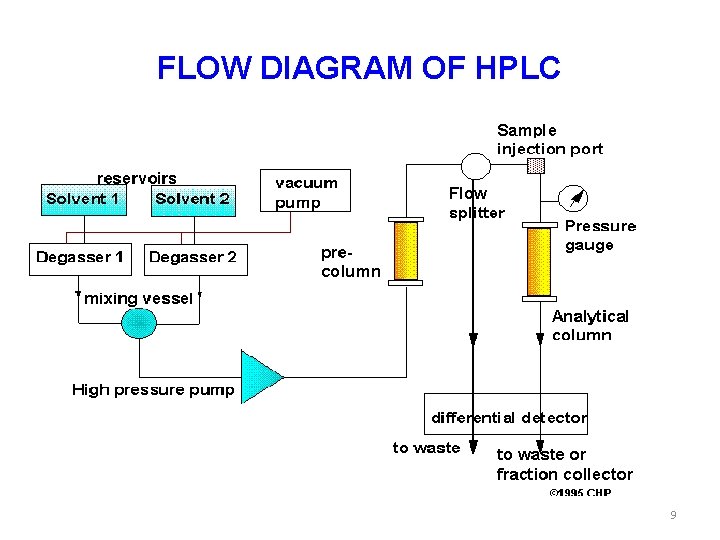
FLOW DIAGRAM OF HPLC 9

Types of column packing in HPLC(particle size distribution) Porous , polymeric bed Porous layer bed Totally porous silica particle Based on styrene-divinyl (diameter 30 -55μm ) polymer consist of thin shell(13μ) of silica on inert core(glass bed ) (diameter <10μm ) with narrow particle size Use in ion exchange & size exclusion After development totally micro particulate packing. Particle (Diameter >20μm are dry packed Particle(diameter <20μm are slurry packed Now , they replaced by silica based packing which are mechanically stable & effective These have not used in HPLC Use in HPLC 10

LC PACKING MATERIAL Pellicular partical • Spherical, nonporous, glass or polymer beads • 30 -40 -μm diameter • Thin porous layer of silica, alumina, or ion-exchange resin deposited on surface • Use in Guard column & (GLC) Porous particle (material) • Most common use in HPLC • 3 -10 -μm diameter • Silica (most common), alumina, or ion-exchange resin • Thin organic film bonded to surface • Addition coating may applied for adsorption • (the former consist of sperical glass or polymer bed with typical 30 -40 -μm diameter ) 11

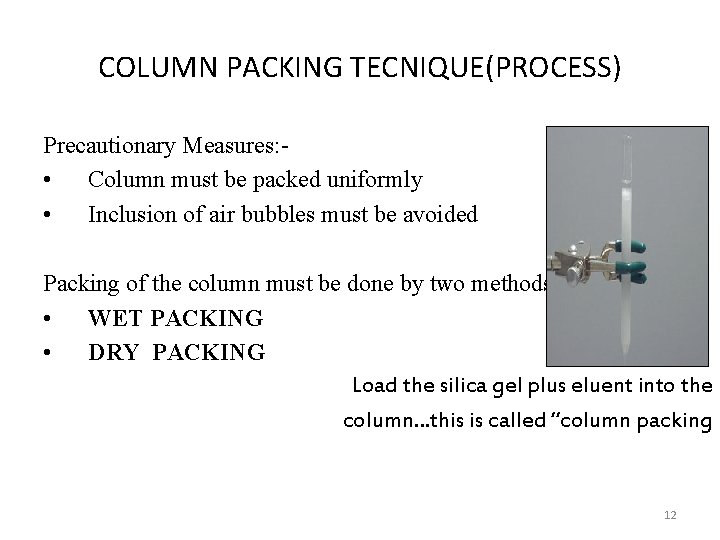
COLUMN PACKING TECNIQUE(PROCESS) Precautionary Measures: • Column must be packed uniformly • Inclusion of air bubbles must be avoided Packing of the column must be done by two methods: • WET PACKING • DRY PACKING Load the silica gel plus eluent into the column…this is called “column packing 12

DRY PACKING METHOD • Dry packing material filled in small amount & deposit by tapping the wall vibrating column • Column be unclamped now tapped on surface • Particle size : 30 -40μ • Linear elution velocity : 2 cm/s • S. p. : plate height: 1 mm • Lower plate height: 0. 2 -0. 5 mm 13

WET OR SLURRY PACKING METHOD • Viscosity method use for to pack analytical column. • Balance density method: suspension of silica gel method • Combination of viscosity & balance method: • • Particle size : 10μ Linear elution velocity : 1 cm/s S. p. : plate height: below 0. 5 mm Lower plate height: 0. 025 mm 14

Procedure for packing of HPLC column with modified silica gel • Stir packing material with 20 ml of toluene – dioxane(1: 1) mix sonicate for 2 min add 15 ml cyclohexanol sonicate for 15 min • If it warm cool it again sonicate 30 min • Pour suspension inyo packing tube • Pump switch on • Pressure increase up to 5600 psi • After passing suspension through column pressure is reduced. • pressure is reduced up to zero • Column closed with end fitting 15

PACKING OF SILICA. Bonded phase packing: pure silica gel particales onto which organic group chemicallly attached. Ex. chlorooctadecyl silane with Oh group of silica gel. Used in partition of closely related compound. R si. OH + CL-Si-R = R chloro Octadecyl R Si-O-Si-R R 16

column packing used for in HPLC column • Increased efficiency & improve analysis time wih suiable material which improve diffusion rate. • Porous adsorbent (>30μ) poor mass taansfer , it can be increase using pellicular support of silica(ex. Zipax, corasil) • the most effective HPLC packing arebased on porous partical with diameter 5μ ttt 5 • Pellicular particale good mechanically stability& shorter diffusion time • Only porous material used in hplc because Pellicular partical low capacity • Rpc are use 17

Mechanism of reverse-phase HPLC (cont) a. Totally Porous packing is dominated by diffusive pores. The surface area of the particle is contained within the pores. A reduction in particle size improves both the interparticle mass transfer and the intraparticle mass transfer. In a porous particle, solutes transfer from moving mobile phase into the stagnant mobile phase within the pores to interact with the stationary phase, then solute molecule must diffuse out of the particle and continue its journey down the column. Such a transfer occurs as the differential separation process proceeds and the solute is eluted from the column. 18

b. The diffusive pores are the same type present in the porous particles, the through - pores allow mobile phase to pass through the packing increasing the rate of mass transfer in mobile phase. When compared with a porous packing of the same particle and pore size, perfusion packings give better efficiency for large molecules c. Nonporous packings (1. 5– 2. 5 μm) allows faster rates (few minutes) of mass transfer and separations for both large and small molecules. Unfortunately, the thin layer of stationary phase limits the capacity of the packing, making it unsuitable for preparative separations. d. Superficially porous packings are similar to nonporous silica but the particle size (5 μm) and surface area are larger resulting in lower pressure drop and increased sample capacity. Recommended for larger biomolecules. 19

RETENTION MECHANISM (RPC) • reversed-phase chromatography the stationary phase is nonpolar, and the mobile phase is polar. • Typical stationary phases are: ong-chain hydrocarbons (C 2, C 8, C 18) attached to a silica support long-chain hydrocarbons (C 2, C 8, C 18) attached to an inert polymer (e. g. monolithic poly-(styrene-codivinylbenzene) support • Typical mobile phases re: • acetonitrile/water mixtures (low viscosity, electron donator) • methanol/water mixtures (potential hydrogen bonding, electron withdrawer) • tetrahydrofuran (increases solvent strength ) 20

• Advantages of reversed-phase chromatography • 90% of all low-molecular weight samples are carried out using a reversed-phase method • good results are obtained for many compounds with few technical complications • rapid equilibration of the column with aqueous mobile phase • Disadvantages of reversed-phase chromatography • only small role in analysis of high-molecular weight compounds • secondary interactions (ion interactions) can cause a bad peak • shape or non-reproducible retentions • p. H influences due to free silanol groups (best derivatization only • removes about 50% of silanol groups due to steric hindrance 21

SOLEVENT In RP chromatography, water is the weak solvent, and acetonitrile, the strong solvent is added gradually to generate a gradient. • Acetonitrile. • Isopropanol. • Methanol. • Ethanol Acetonitrile is the reverse phase solvent of choice because the UV cut offer acetonitrile is 190 nm, allowing detection at lower wavelengths. It is less viscous than methanol, thus causing less fluctuations in pressure. Less buble formation occurs when it is mixed with water. It has also better selectivity for peptides and proteins. Isopropanol is used either alone or in combination with acetonitrile to elute large or hydrophobic proteins. • 22

Factors affecting to column efficiency • • • Dimension of the column Particle size of column packing Pore diameter of column packing Temperature Quality or Nature of solvents Packing the column 23

Applications of HPLC • Reverse phase partition HPLC is useful for separation of polar compounds such as drug & their metabolites, pepide, vitamin, polyphenol & steroid. • Partition chromatography has powerful tool for the separation of closely related substance. 24

Reference 1)Horbert H Willard, Frank A Settle, Instrumental method of analysis published by saitsh kumar jain CBS publicaion, 1 st edition, p. n. 614 -16 2)B. K. Sharma, Instrumental method of chemical analysis, publishedby. KRISHNAprakashan. Media pvt ltd 22 ndedition, 2005, p. n. c-298 -300 3)G. R. Chatwal & S. K. Anand, Instrumental method of chemical analysis, published by himalaya publication house mumbai, 5 thedition, 2007, p. n. 2. 626, 2. 638. 4)P. D. Shetthi HPLC , Quantitative analysis of pharmaceutical formulation, CBS publisher & distributors, 1 st edition, 2006, p. n. 59 -60. 25

Thank you 26