Coloring the Periodic Table Groups 8 th Grade

Coloring the Periodic Table Groups 8 th Grade Science Mrs. Dickerson Some images are from www. chem 4 kids. com www. middleschoolscience. com 2008
Groups on the Periodic Table Elements on the periodic table can be grouped based on their chemical properties. Each group has a specific name to differentiate it from the other groups in the periodic table. Elements in each group react differently with other elements.
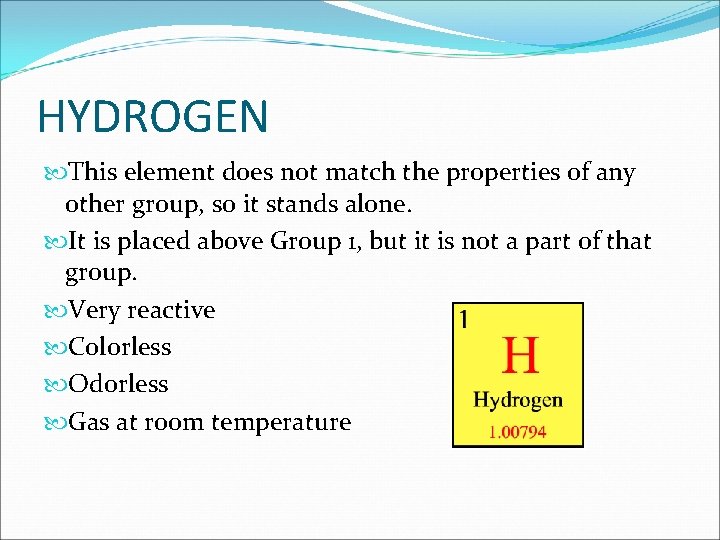
HYDROGEN This element does not match the properties of any other group, so it stands alone. It is placed above Group 1, but it is not a part of that group. Very reactive Colorless Odorless Gas at room temperature
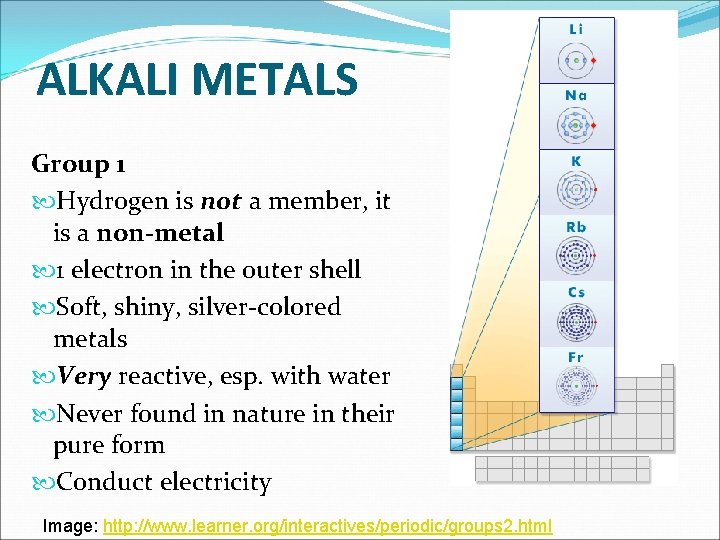
ALKALI METALS Group 1 Hydrogen is not a member, it is a non-metal 1 electron in the outer shell Soft, shiny, silver-colored metals Very reactive, esp. with water Never found in nature in their pure form Conduct electricity Image: http: //www. learner. org/interactives/periodic/groups 2. html

Alkali Metals Examples Lithium Sodium
ALKALINE-EARTH METALS Group 2 2 electrons in the outer shell White or silvercolored and malleable (easily shaped) Reactive, but less than Alkali metals Conduct electricity

Alkaline-Earth Metals Examples Calcium
TRANSITION METALS Groups 3 - 12 Good conductors of heat and electricity. Some are used for jewelry. The transition metals are able to put up to 32 electrons in their second to last shell. Shiny

Transition Metals Examples
BORON GROUP Group 13 3 electrons in the outer shell 4 are metals Boron is a metalloid

Boron Group Examples Aluminum
CARBON GROUP Group 14 4 electrons in the outer shell Contains 2 metals, 2 metalloids, and one nonmetal

Carbon Group Examples Carbon Fiber
NITROGEN GROUP Group 15 5 electrons in the outer shell Can share electrons to form compounds Contains 1 metal, two metalloids, and two nonmetals

Nitrogen Group Examples Nitrogen is found in fertilizers Liquid Nitrogen
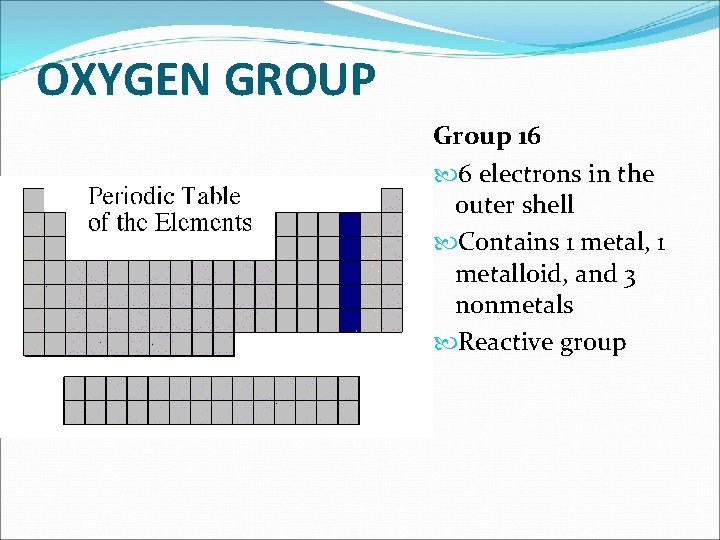
OXYGEN GROUP Group 16 6 electrons in the outer shell Contains 1 metal, 1 metalloid, and 3 nonmetals Reactive group

Oxygen Group Examples Sulfur is found in gunpowder
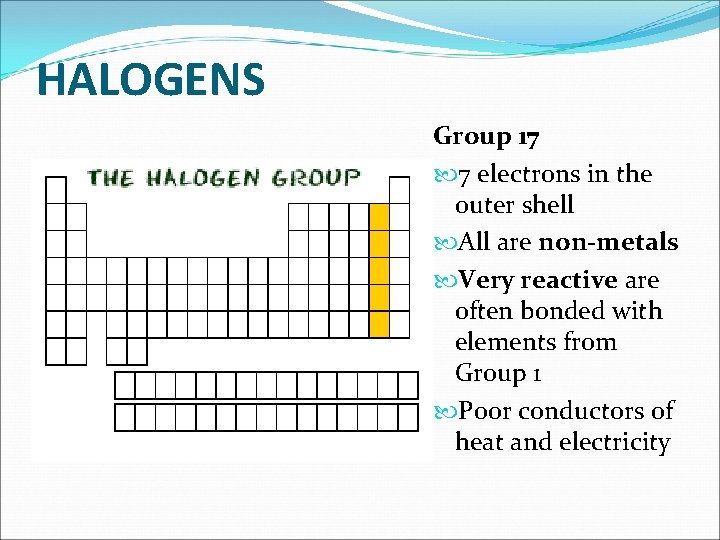
HALOGENS Group 17 7 electrons in the outer shell All are non-metals Very reactive are often bonded with elements from Group 1 Poor conductors of heat and electricity

Halogen Group Examples
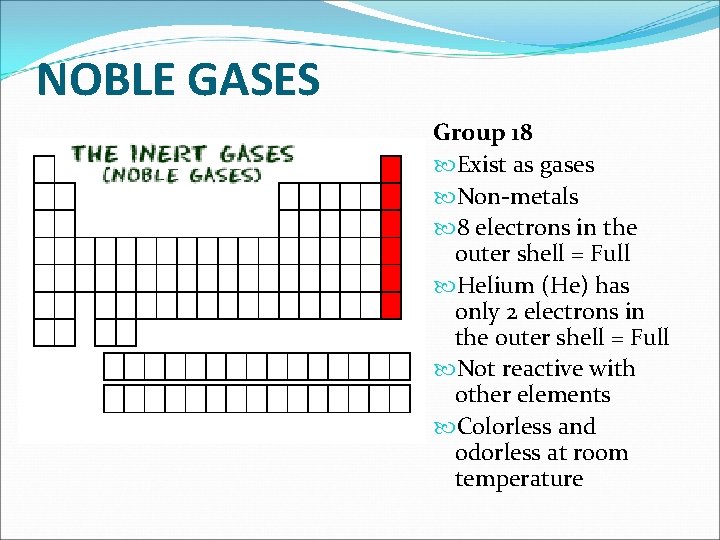
NOBLE GASES Group 18 Exist as gases Non-metals 8 electrons in the outer shell = Full Helium (He) has only 2 electrons in the outer shell = Full Not reactive with other elements Colorless and odorless at room temperature

Noble Gases Examples
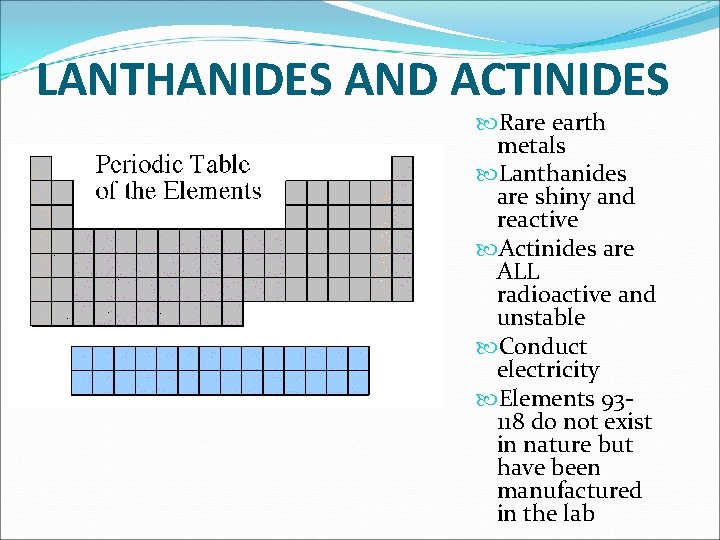
LANTHANIDES AND ACTINIDES Rare earth metals Lanthanides are shiny and reactive Actinides are ALL radioactive and unstable Conduct electricity Elements 93118 do not exist in nature but have been manufactured in the lab

LANTHANIDES AND ACTINIDES

THE END.
- Slides: 24