Coloring Part 1 Coloring the Periodic Table Families
Coloring Part 1 Coloring the Periodic Table Families Discovery Science
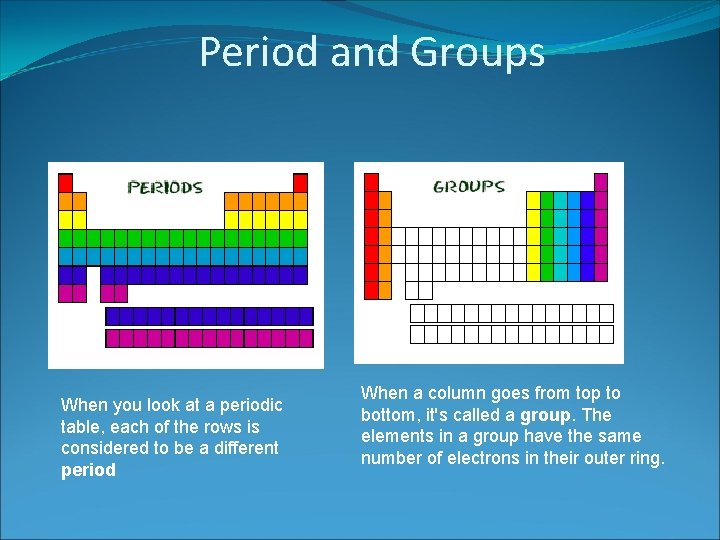
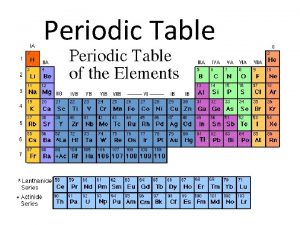
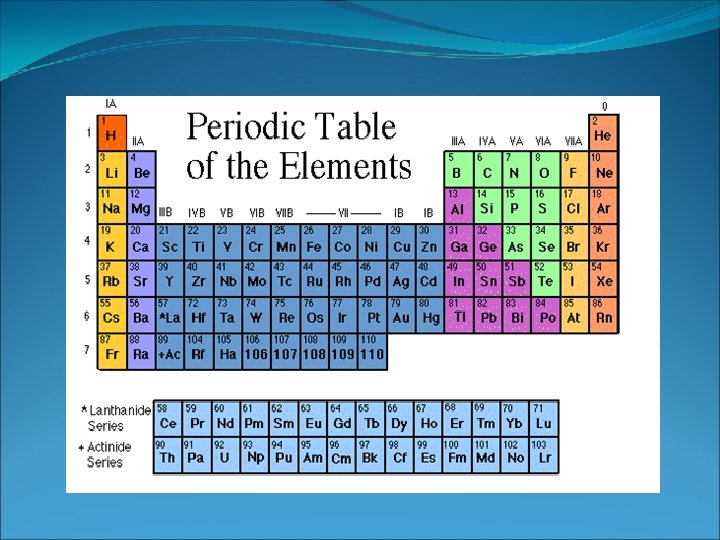
Period and Groups When you look at a periodic table, each of the rows is considered to be a different period When a column goes from top to bottom, it's called a group. The elements in a group have the same number of electrons in their outer ring.
Families on the Periodic Table Elements on the periodic table can be grouped into families bases on their chemical properties. Each family has a specific name to differentiate it from the other families in the periodic table. Elements in each family react differently with other elements. Remember Patty Proton and Her relatives…not all of us Get along with all of our Relatives.
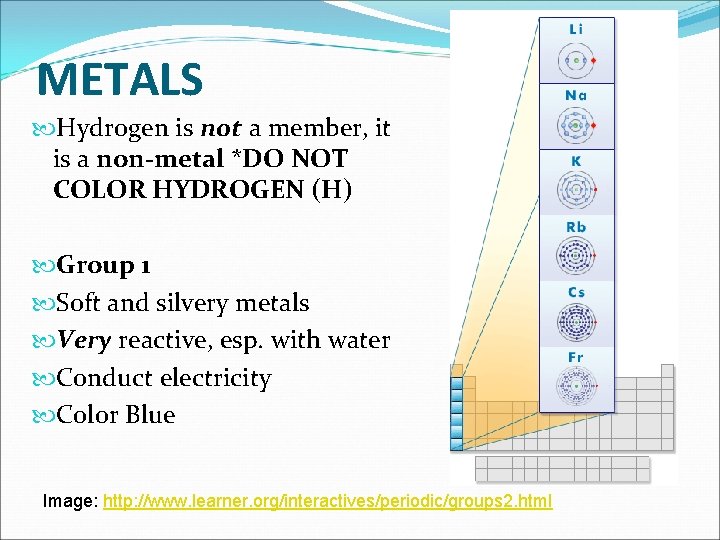
METALS Hydrogen is not a member, it is a non-metal *DO NOT COLOR HYDROGEN (H) Group 1 Soft and silvery metals Very reactive, esp. with water Conduct electricity Color Blue Image: http: //www. learner. org/interactives/periodic/groups 2. html
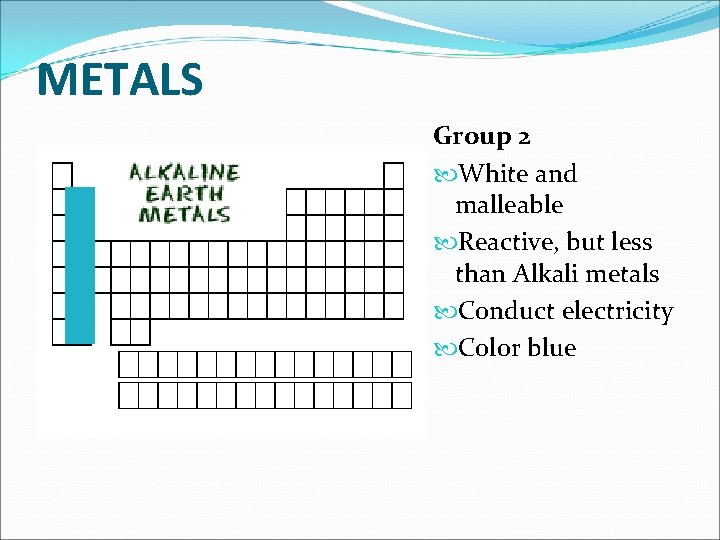
METALS Group 2 White and malleable Reactive, but less than Alkali metals Conduct electricity Color blue
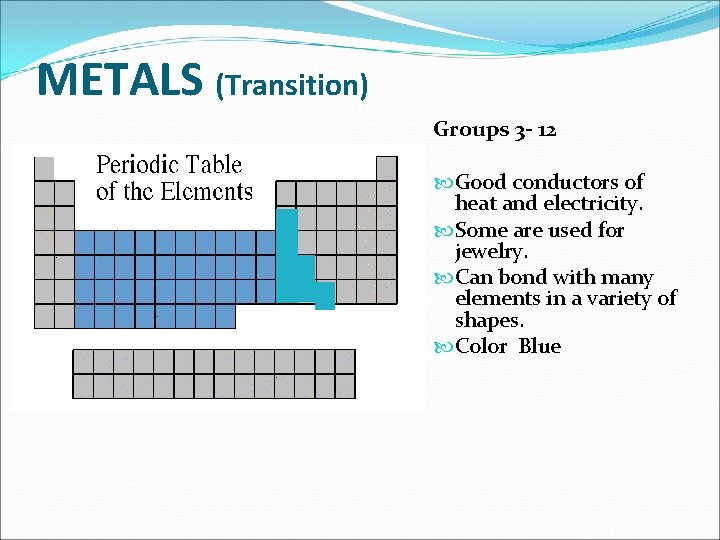
METALS (Transition) Groups 3 - 12 Good conductors of heat and electricity. Some are used for jewelry. Can bond with many elements in a variety of shapes. Color Blue
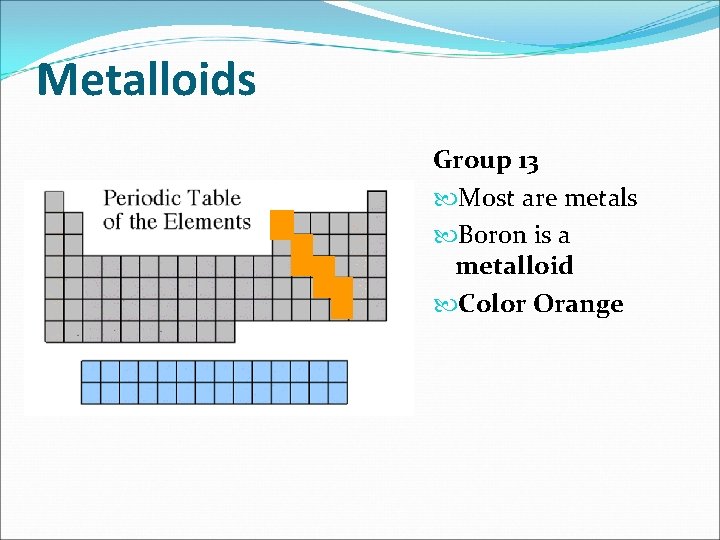
Metalloids Group 13 Most are metals Boron is a metalloid Color Orange
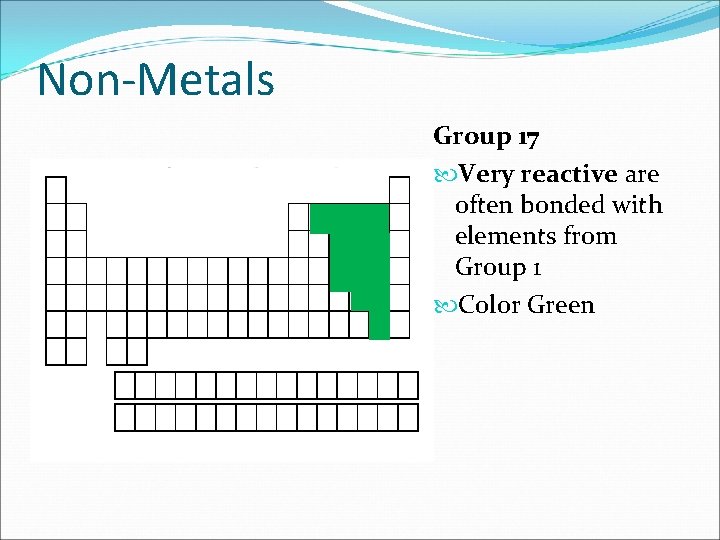
Non-Metals Group 17 Very reactive are often bonded with elements from Group 1 Color Green
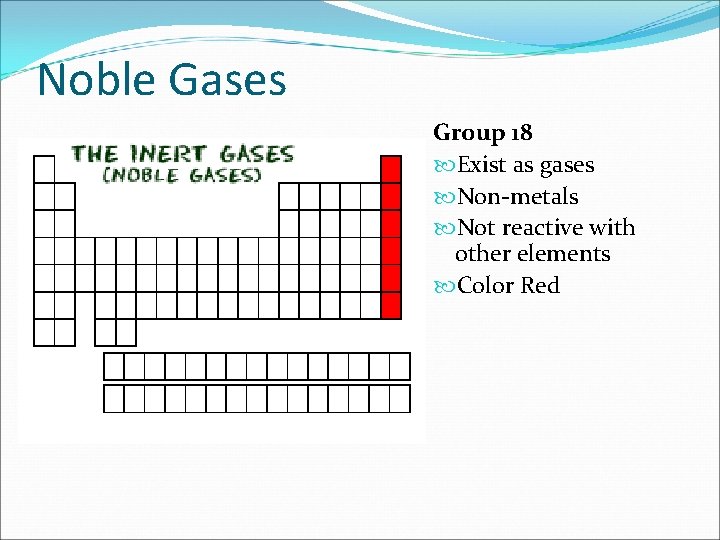
Noble Gases Group 18 Exist as gases Non-metals Not reactive with other elements Color Red
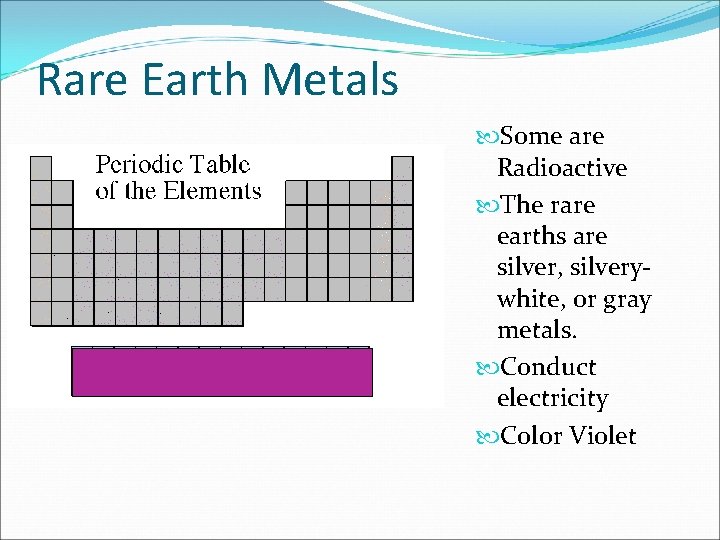
Rare Earth Metals Some are Radioactive The rare earths are silver, silverywhite, or gray metals. Conduct electricity Color Violet
- Slides: 11