Colorectal polyps l Visible protrusion above the surface

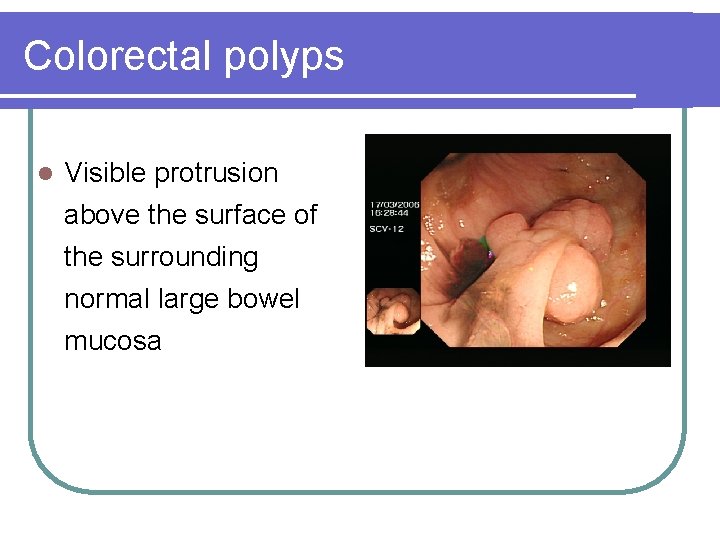
Colorectal polyps l Visible protrusion above the surface of the surrounding normal large bowel mucosa
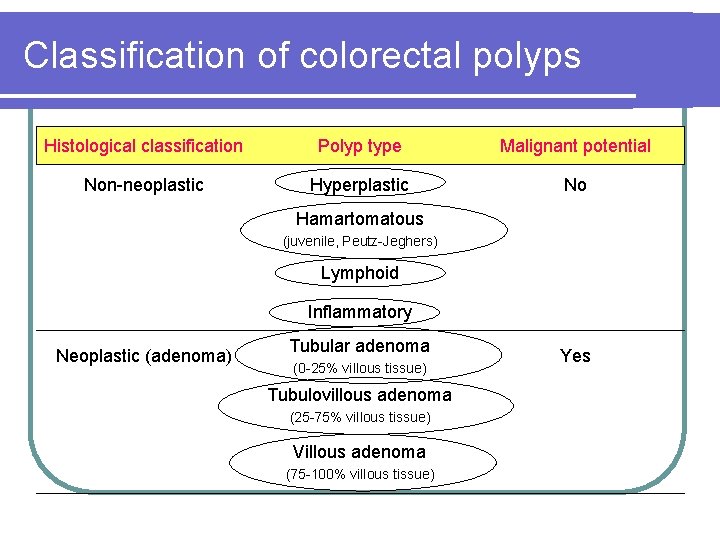
Classification of colorectal polyps Histological classification Polyp type Malignant potential Non-neoplastic Hyperplastic No Hamartomatous (juvenile, Peutz-Jeghers) Lymphoid Inflammatory Neoplastic (adenoma) Tubular adenoma (0 -25% villous tissue) Tubulovillous adenoma (25 -75% villous tissue) Villous adenoma (75 -100% villous tissue) Yes
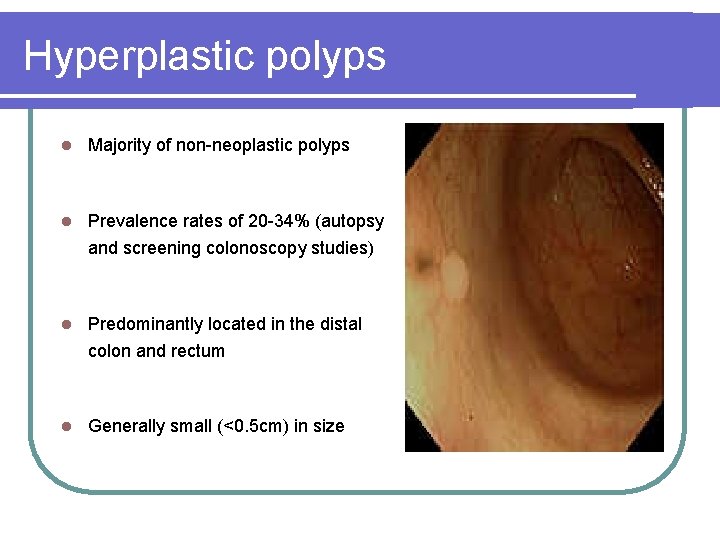
Hyperplastic polyps l Majority of non-neoplastic polyps l Prevalence rates of 20 -34% (autopsy and screening colonoscopy studies) l Predominantly located in the distal colon and rectum l Generally small (<0. 5 cm) in size
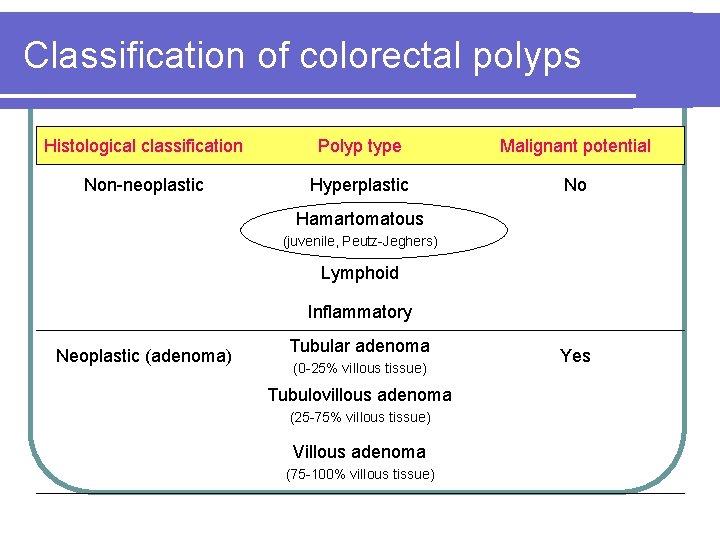
Classification of colorectal polyps Histological classification Polyp type Malignant potential Non-neoplastic Hyperplastic No Hamartomatous (juvenile, Peutz-Jeghers) Lymphoid Inflammatory Neoplastic (adenoma) Tubular adenoma (0 -25% villous tissue) Tubulovillous adenoma (25 -75% villous tissue) Villous adenoma (75 -100% villous tissue) Yes

Hamartomatous polyposis syndromes l Juvenile polyps l Peutz-Jeghers polyps l Cronkhite-Canada syndrome
Juvenile polyposis l Presence of 10 juvenile polyps in the GI tract l Incidence: 1 in 100, 000 persons l Autosomal dominant l Mutation of SMAD 4 gene on chromosome 18
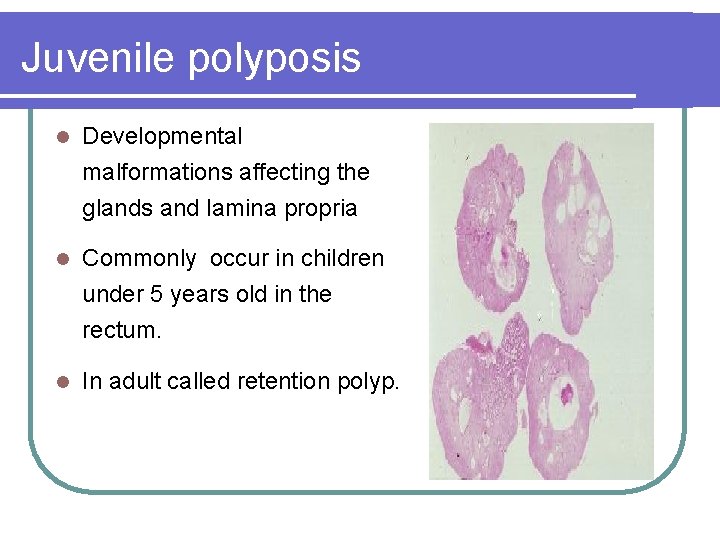
Juvenile polyposis l Developmental malformations affecting the glands and lamina propria l Commonly occur in children under 5 years old in the rectum. l In adult called retention polyp.
Juvenile polyposis l Colon cancer risk 50% l Risk of gastric, duodenal, and pancreatic cancers
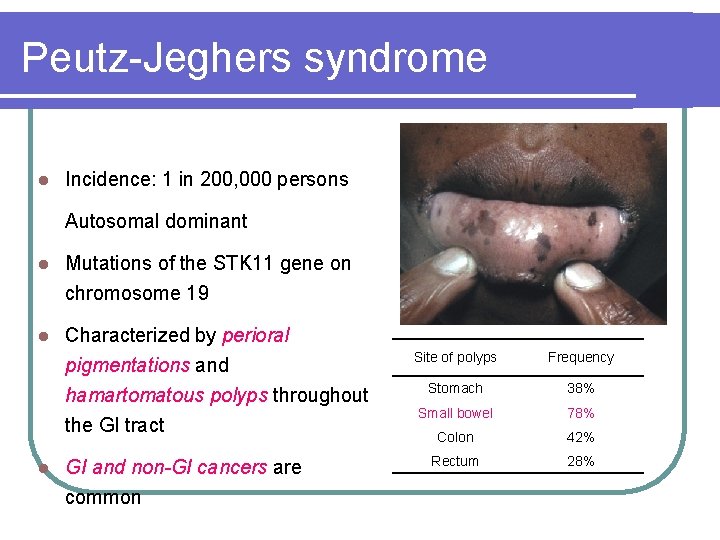
Peutz-Jeghers syndrome l Incidence: 1 in 200, 000 persons Autosomal dominant l Mutations of the STK 11 gene on chromosome 19 l Characterized by perioral pigmentations and hamartomatous polyps throughout the GI tract l GI and non-GI cancers are common Site of polyps Frequency Stomach 38% Small bowel 78% Colon 42% Rectum 28%
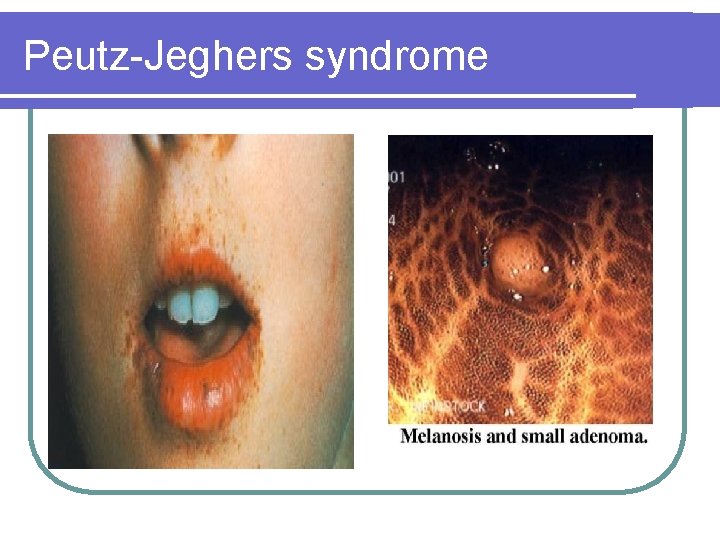
Peutz-Jeghers syndrome
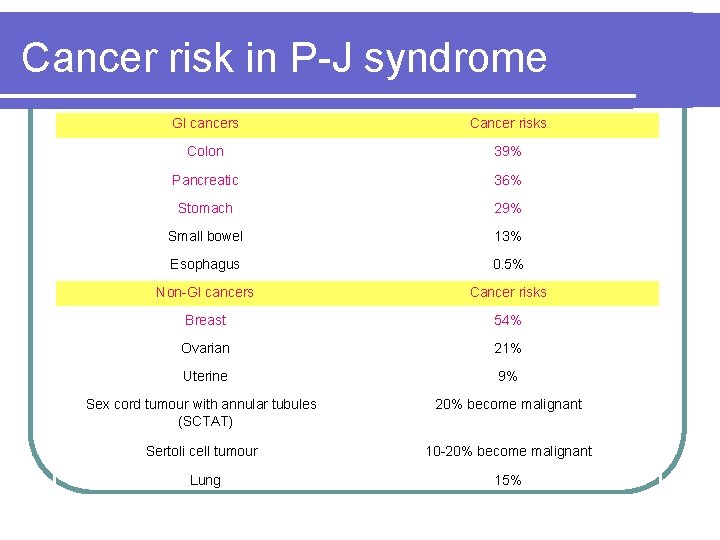
Cancer risk in P-J syndrome GI cancers Cancer risks Colon 39% Pancreatic 36% Stomach 29% Small bowel 13% Esophagus 0. 5% Non-GI cancers Cancer risks Breast 54% Ovarian 21% Uterine 9% Sex cord tumour with annular tubules (SCTAT) 20% become malignant Sertoli cell tumour 10 -20% become malignant Lung 15%

Cronkhite-Canada syndrome l Gastrointestinal hamartomatous polyposis that lead to l l Diarrhea, Weight loss and Abdominal pain Extra-intestinal manifestations l l l Alopecia, Cutaneous hyperpigmentation, Onycho-dystrophy

Cronkhite-Canada syndrome l Five-year mortality rates as high as 55 percent have been reported with most deaths due to gastrointestinal bleeding, l sepsis, and l congestive heart failure. l l Treatment has included nutritional support, corticosteroids, acid suppression, and antibiotics
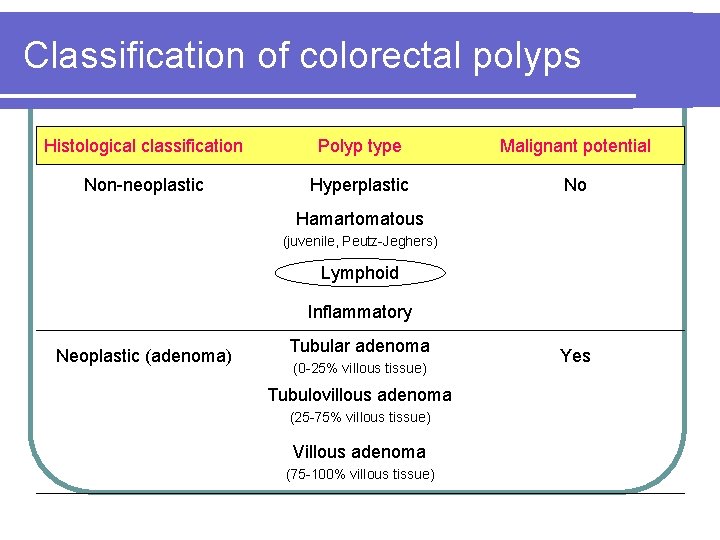
Classification of colorectal polyps Histological classification Polyp type Malignant potential Non-neoplastic Hyperplastic No Hamartomatous (juvenile, Peutz-Jeghers) Lymphoid Inflammatory Neoplastic (adenoma) Tubular adenoma (0 -25% villous tissue) Tubulovillous adenoma (25 -75% villous tissue) Villous adenoma (75 -100% villous tissue) Yes
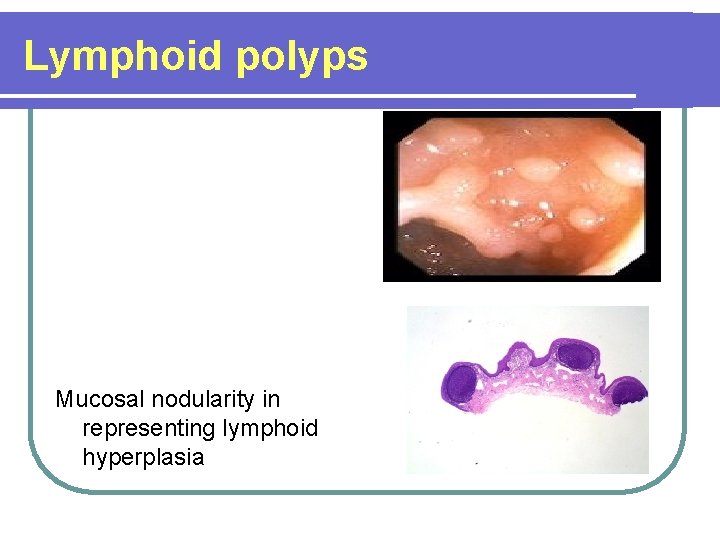
Lymphoid polyps Mucosal nodularity in representing lymphoid hyperplasia
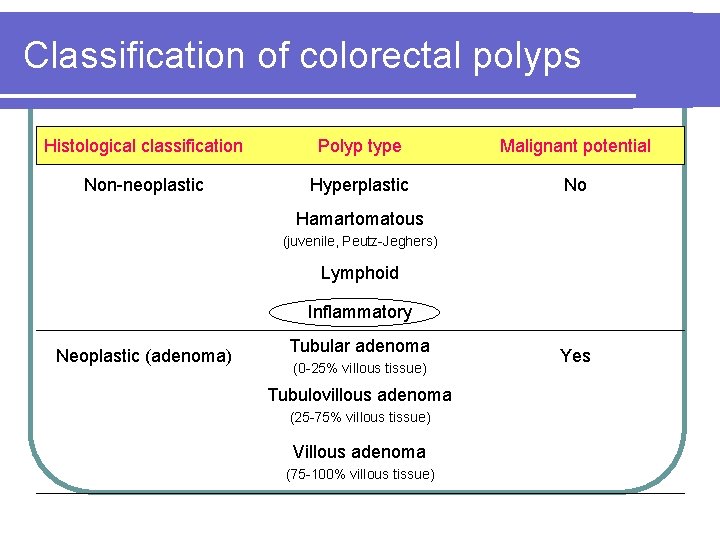
Classification of colorectal polyps Histological classification Polyp type Malignant potential Non-neoplastic Hyperplastic No Hamartomatous (juvenile, Peutz-Jeghers) Lymphoid Inflammatory Neoplastic (adenoma) Tubular adenoma (0 -25% villous tissue) Tubulovillous adenoma (25 -75% villous tissue) Villous adenoma (75 -100% villous tissue) Yes
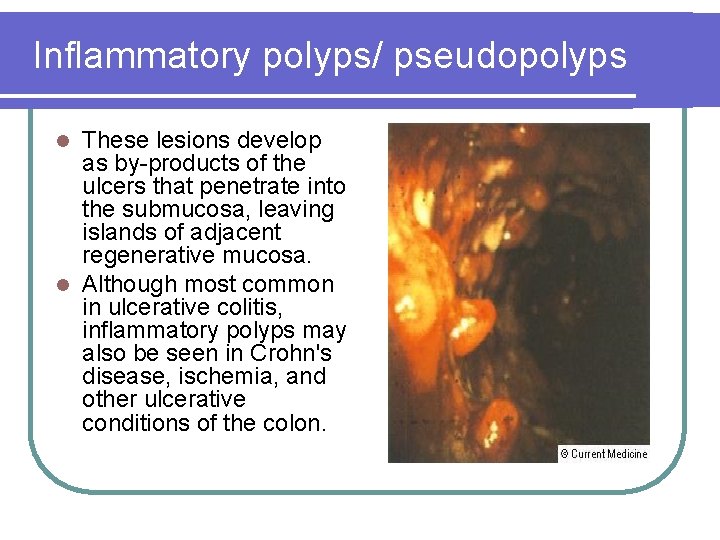
Inflammatory polyps/ pseudopolyps These lesions develop as by-products of the ulcers that penetrate into the submucosa, leaving islands of adjacent regenerative mucosa. l Although most common in ulcerative colitis, inflammatory polyps may also be seen in Crohn's disease, ischemia, and other ulcerative conditions of the colon. l
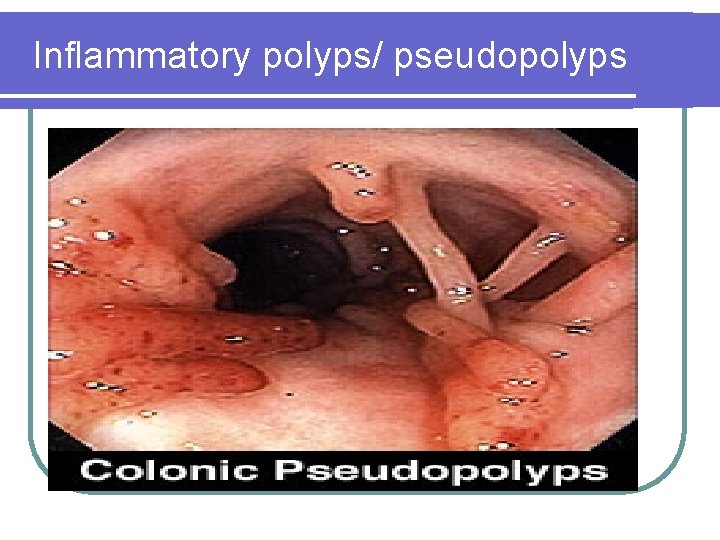
Inflammatory polyps/ pseudopolyps
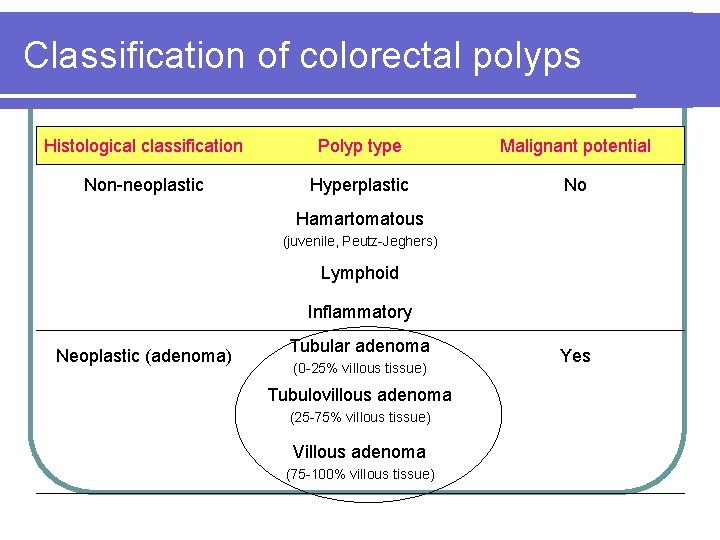
Classification of colorectal polyps Histological classification Polyp type Malignant potential Non-neoplastic Hyperplastic No Hamartomatous (juvenile, Peutz-Jeghers) Lymphoid Inflammatory Neoplastic (adenoma) Tubular adenoma (0 -25% villous tissue) Tubulovillous adenoma (25 -75% villous tissue) Villous adenoma (75 -100% villous tissue) Yes
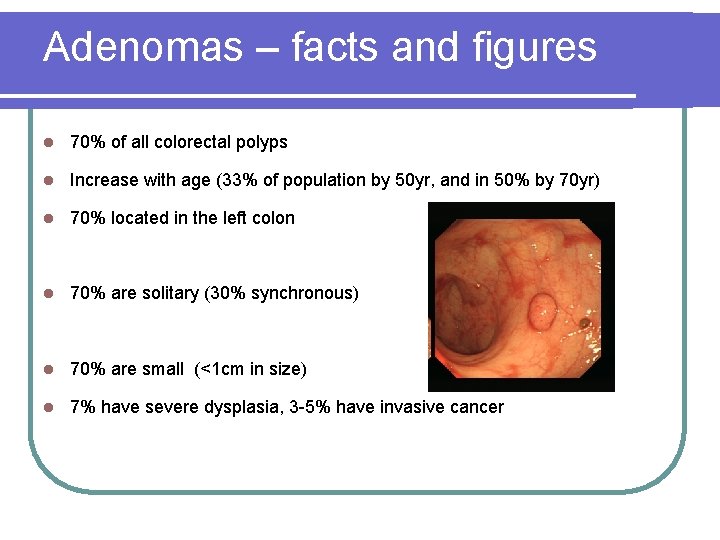
Adenomas – facts and figures l 70% of all colorectal polyps l Increase with age (33% of population by 50 yr, and in 50% by 70 yr) l 70% located in the left colon l 70% are solitary (30% synchronous) l 70% are small (<1 cm in size) l 7% have severe dysplasia, 3 -5% have invasive cancer
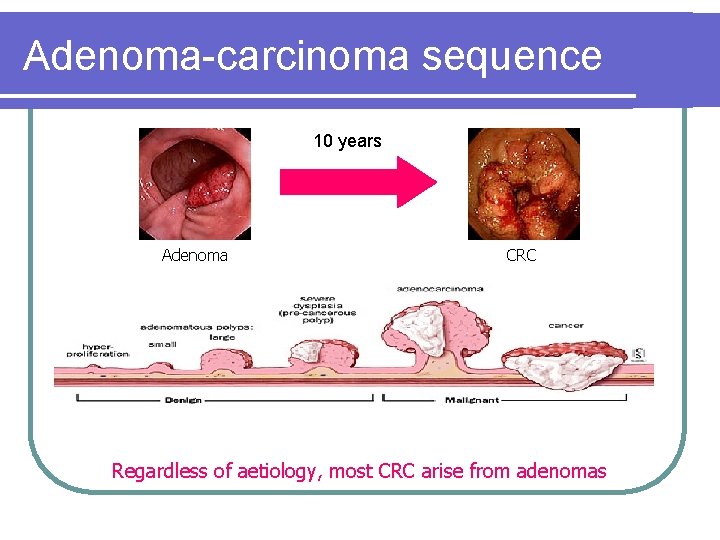
Adenoma-carcinoma sequence 10 years Adenoma CRC Regardless of aetiology, most CRC arise from adenomas
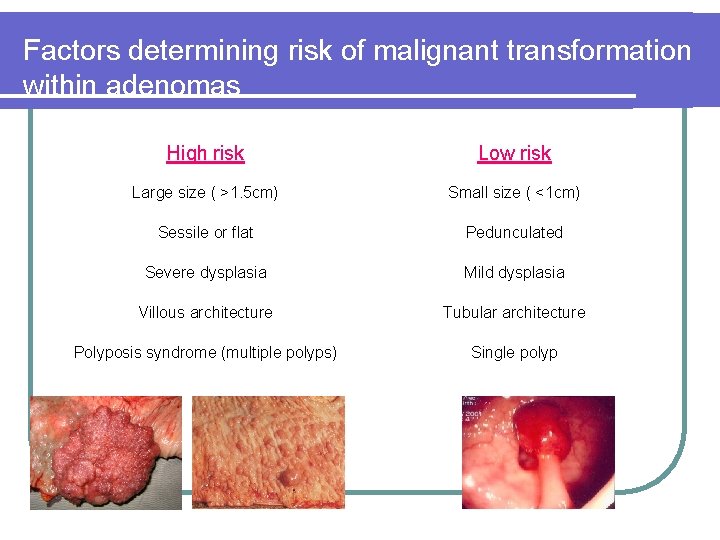
Factors determining risk of malignant transformation within adenomas High risk Low risk Large size ( >1. 5 cm) Small size ( <1 cm) Sessile or flat Pedunculated Severe dysplasia Mild dysplasia Villous architecture Tubular architecture Polyposis syndrome (multiple polyps) Single polyp
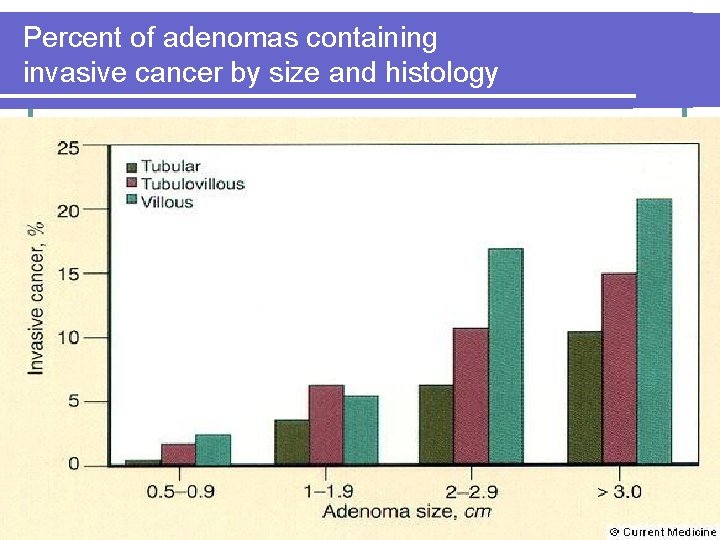
Percent of adenomas containing invasive cancer by size and histology
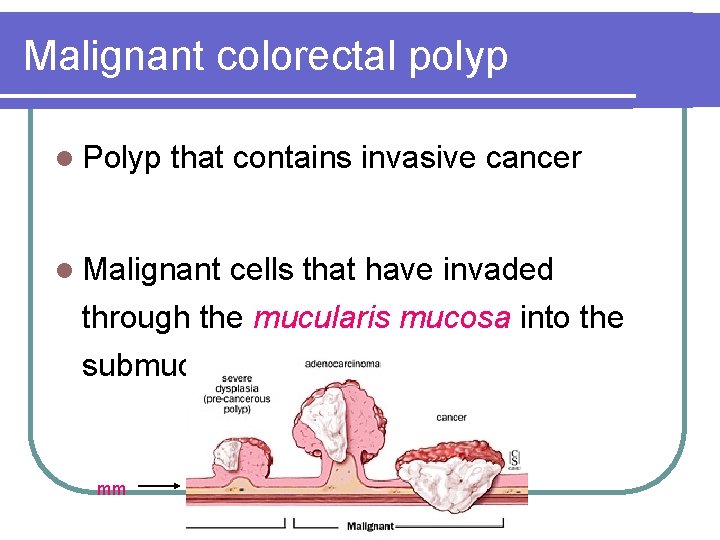
Malignant colorectal polyp l Polyp that contains invasive cancer l Malignant cells that have invaded through the mucularis mucosa into the submucosa mm
Familial adenomatous polyposis (FAP) l 1% of all CRC l Present in about 1 in 8000 births l Autosomal dominant with near 100% penetrance
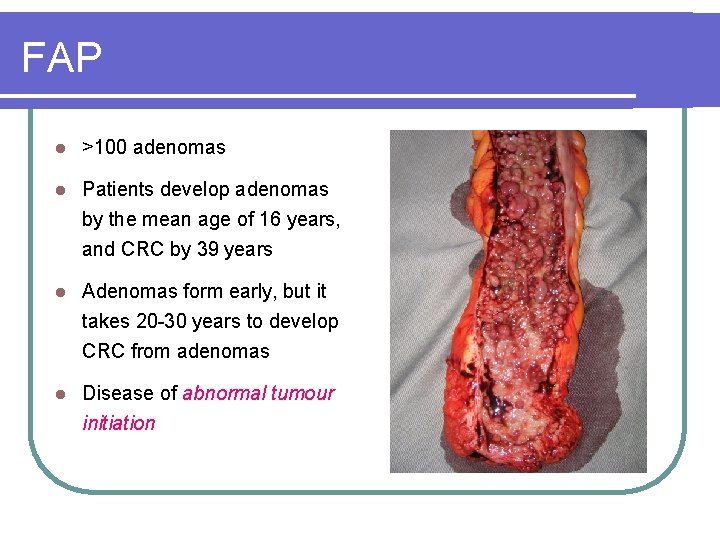
FAP l >100 adenomas l Patients develop adenomas by the mean age of 16 years, and CRC by 39 years l Adenomas form early, but it takes 20 -30 years to develop CRC from adenomas l Disease of abnormal tumour initiation
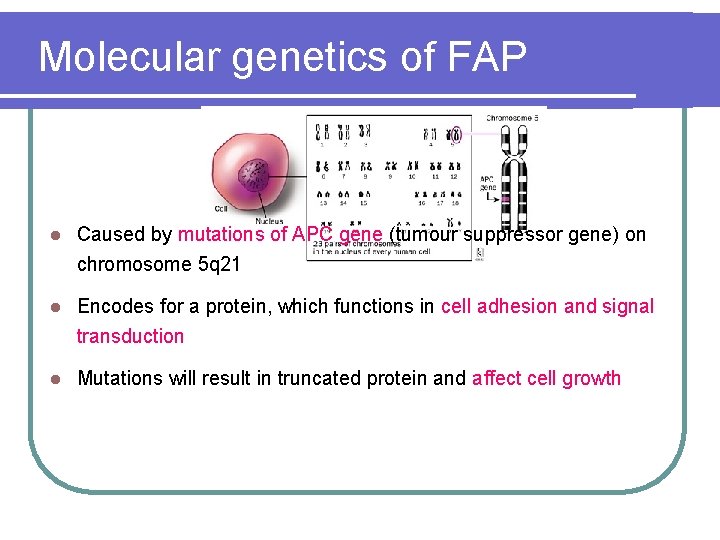
Molecular genetics of FAP l Caused by mutations of APC gene (tumour suppressor gene) on chromosome 5 q 21 l Encodes for a protein, which functions in cell adhesion and signal transduction l Mutations will result in truncated protein and affect cell growth
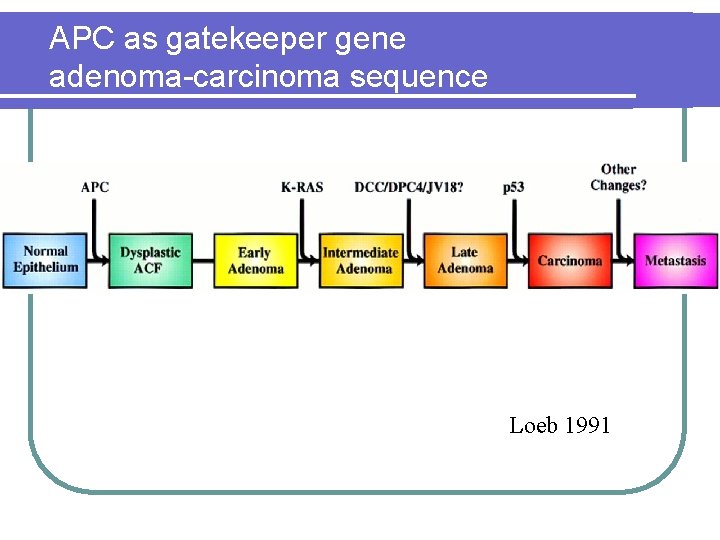
APC as gatekeeper gene adenoma-carcinoma sequence Loeb 1991
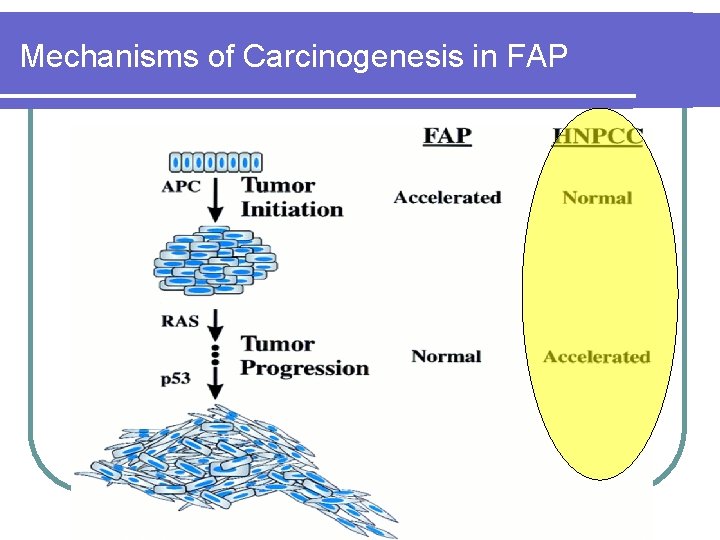
Mechanisms of Carcinogenesis in FAP
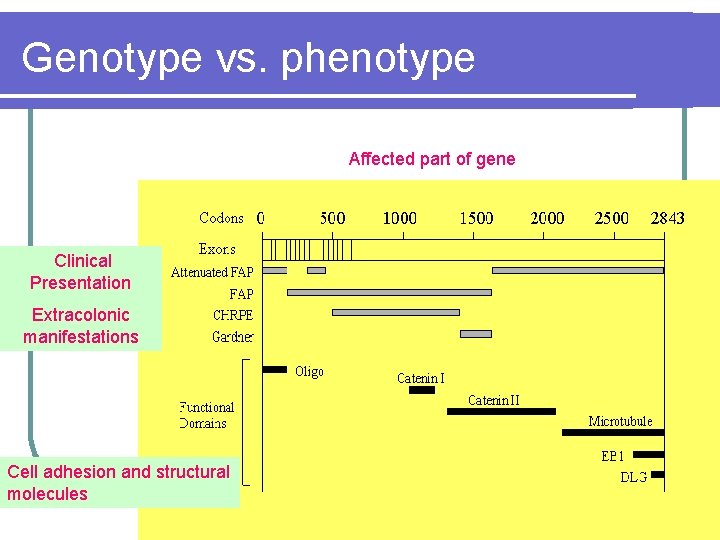
Genotype vs. phenotype Affected part of gene Clinical Presentation Extracolonic manifestations Cell adhesion and structural molecules
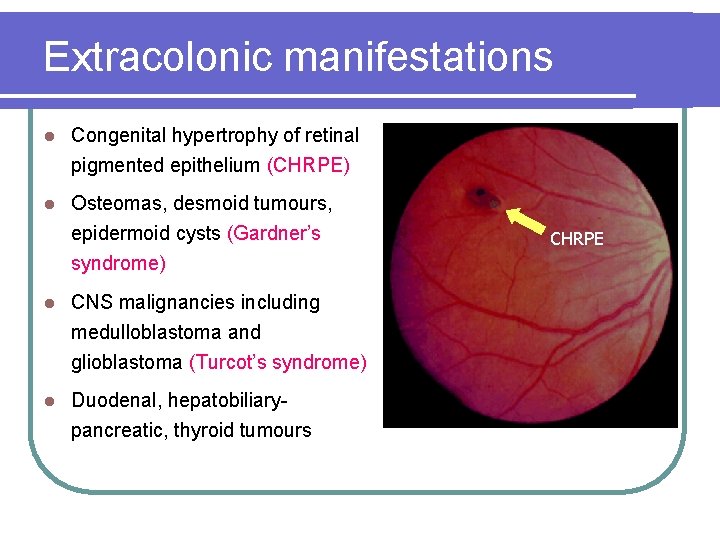
Extracolonic manifestations l Congenital hypertrophy of retinal pigmented epithelium (CHRPE) l Osteomas, desmoid tumours, epidermoid cysts (Gardner’s syndrome) l CNS malignancies including medulloblastoma and glioblastoma (Turcot’s syndrome) l Duodenal, hepatobiliarypancreatic, thyroid tumours CHRPE
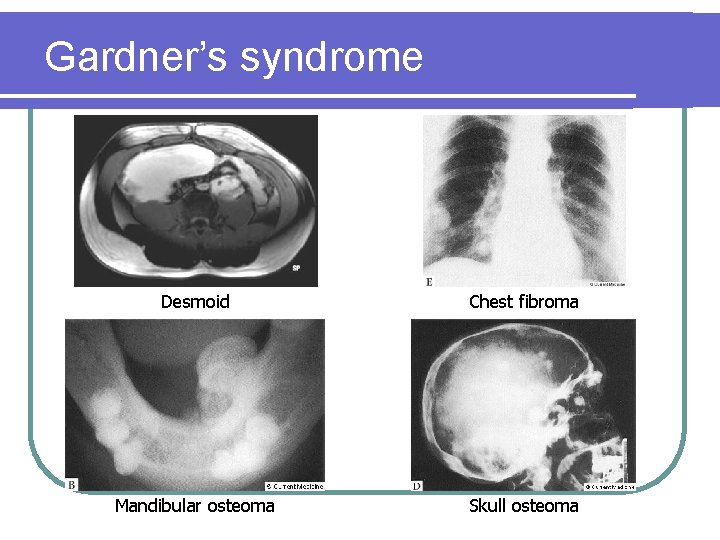
Gardner’s syndrome Desmoid Chest fibroma Mandibular osteoma Skull osteoma
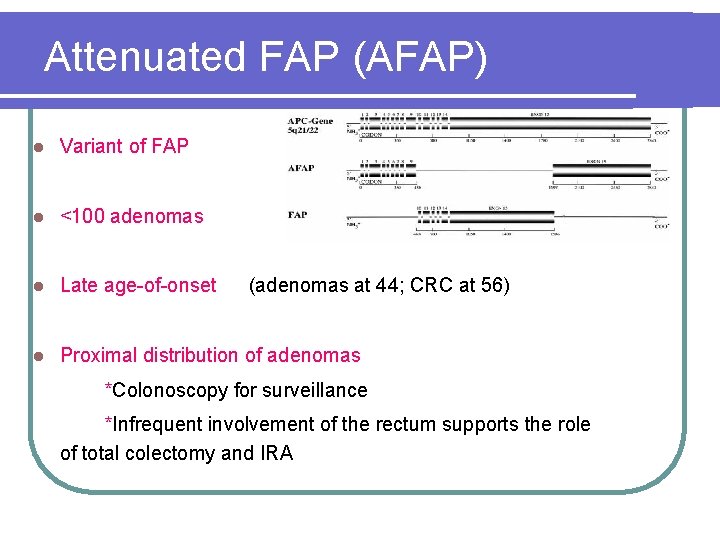
Attenuated FAP (AFAP) l Variant of FAP l <100 adenomas l Late age-of-onset l Proximal distribution of adenomas (adenomas at 44; CRC at 56) *Colonoscopy for surveillance *Infrequent involvement of the rectum supports the role of total colectomy and IRA
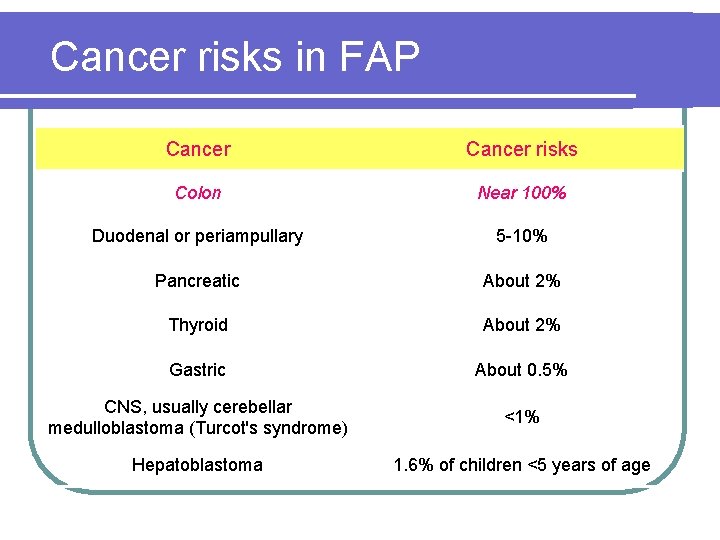
Cancer risks in FAP Cancer risks Colon Near 100% Duodenal or periampullary 5 -10% Pancreatic About 2% Thyroid About 2% Gastric About 0. 5% CNS, usually cerebellar medulloblastoma (Turcot's syndrome) <1% Hepatoblastoma 1. 6% of children <5 years of age
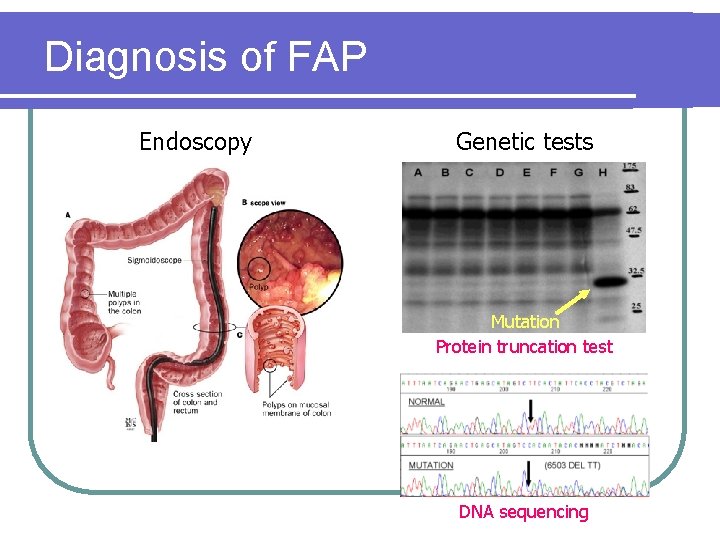
Diagnosis of FAP Endoscopy Genetic tests Mutation Protein truncation test DNA sequencing
Screening of FAP l Genetic screening of family members for APC mutations l Annual flexible sigmoidoscopy beginning at age 10 -12 until age 40, then every 3 -5 years *If polyposis is present, colectomy should be considered l Upper GIT Endoscopy every 1 -3 years is also recommended to evaluate for upper GI adenomas
Hereditary nonpolyposis colorectal cancer (HNPCC) Dr. A. S. Warthin and the first HNPCC pedigree, ‘the family G’ Dr. Henry Lynch first described the term ‘cancer family syndrome’ in 1966 (later 1895 renamed as Lynch syndrome and HNPCC)
HNPCC l 2 -5% of all CRC l Autosomal dominant l 70 -80% penetrance l It takes only 3 -5 years to develop CRC from adenomas Accelerated progression
HNPCC: Lynch syndromes Lynch syndrome II Early onset of CRC (40 -45 years) Features of Lynch Syndrome I + extracolonic malignancies Predominantly proximal to the splenic flexure (60 -70%) Increase frequency of synchronous and metachronous lesions (33%) *Gastric, small bowel, hepatobiliary, endometrial, ovarian, ureteral and renal tumours
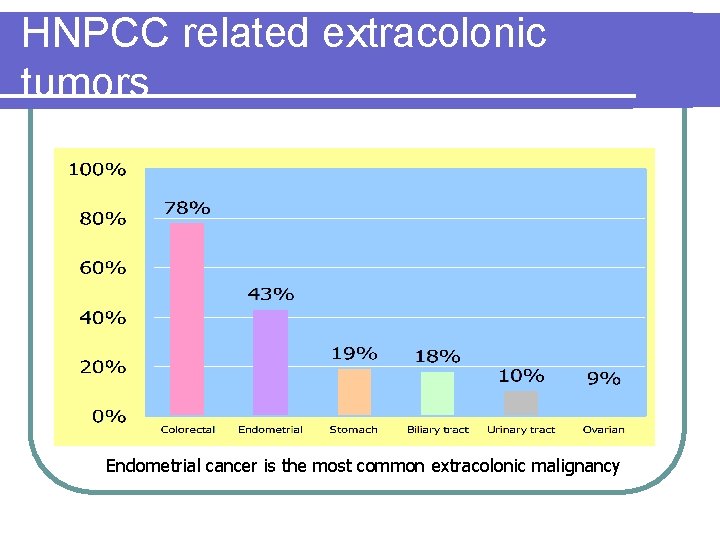
HNPCC related extracolonic tumors Endometrial cancer is the most common extracolonic malignancy
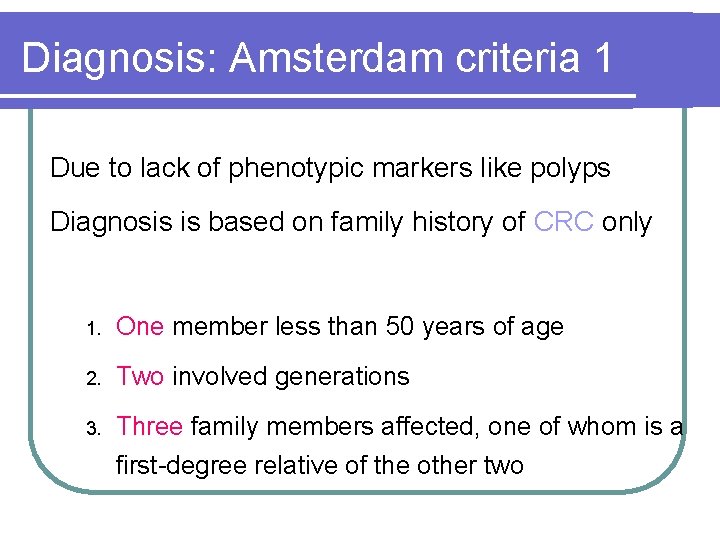
Diagnosis: Amsterdam criteria 1 Due to lack of phenotypic markers like polyps Diagnosis is based on family history of CRC only 1. One member less than 50 years of age 2. Two involved generations 3. Three family members affected, one of whom is a first-degree relative of the other two

Diagnosis: Amsterdam criteria 2 Same as Amsterdam 1 but includes all HNPCC related tumors
Molecular genetics of HNPCC is caused by mutations of DNA mismatch repair (MMR) genes Survey DNA for replication errors
Molecular genetics of HNPCC l Mutations of these MMR genes will result in replication errors during DNA synthesis (microsatellite instability) leading to acceleration of genetic mutations l HNPCC patients develop adenomas at the same rate as the general population l Once these adenomas develop, however, defective DNA repair ensues and mismatches accumulates l Thus, it takes only 3 -5 years to develop CRC from adenomas
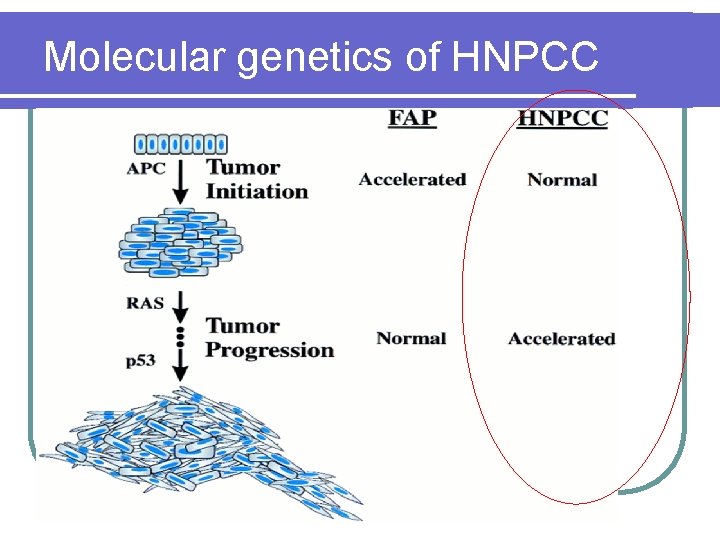
Molecular genetics of HNPCC
Screening of HNPCC l Colonoscopy every 2 years starting at ages 20 -25 or 5 years younger than the earliest diagnosis of CRC whichever is earlier until 40 yr , and then annually l Flexible sigmoidoscopy is not acceptable, due to the proximal location of tumours l Transvaginal US and endometrial aspiration annually starting at ages 25 -35 years are also recommended
Management of colorectal polyps Factors Affecting Location: colon or rectum Number: solitary or multiple Morphology: pedunculated or sessile Histology: benign or malignant
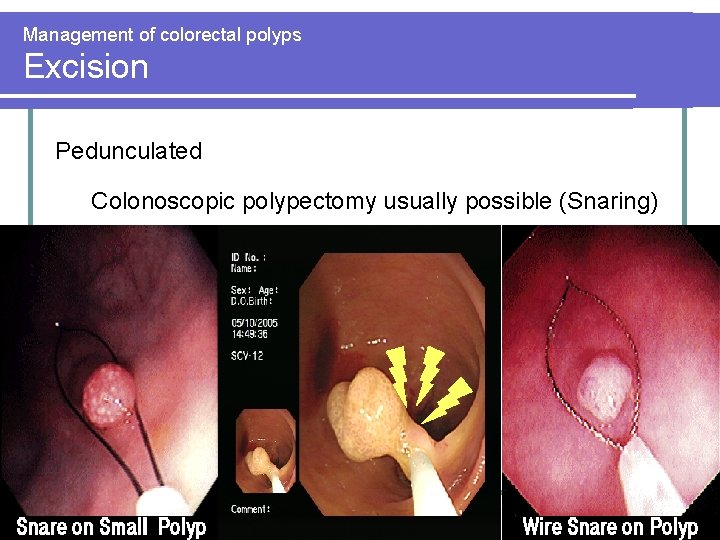
Management of colorectal polyps Excision Pedunculated Colonoscopic polypectomy usually possible (Snaring)
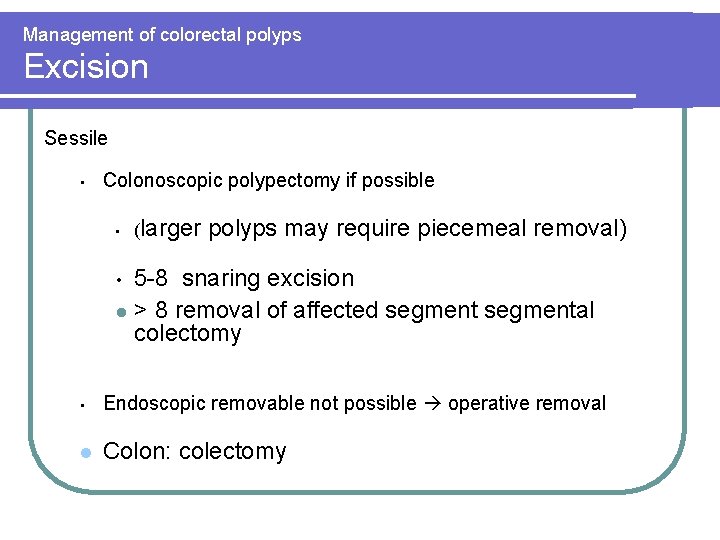
Management of colorectal polyps Excision Sessile • Colonoscopic polypectomy if possible • (larger polyps may require piecemeal removal) 5 -8 snaring excision l > 8 removal of affected segmental colectomy • • Endoscopic removable not possible operative removal l Colon: colectomy
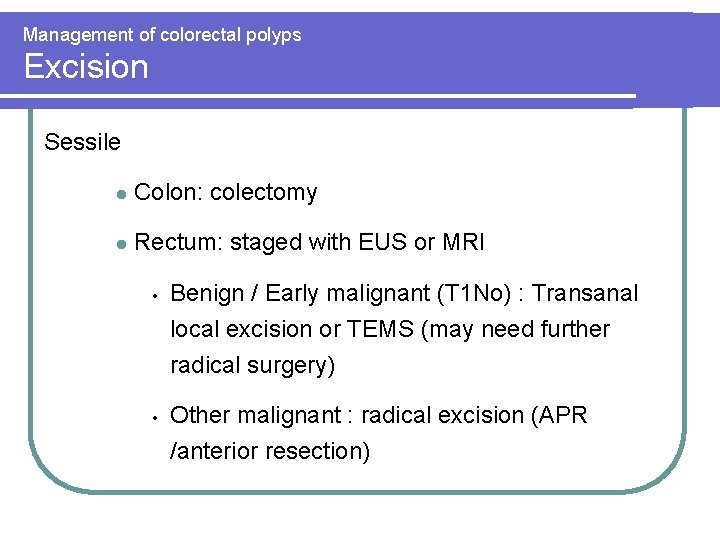
Management of colorectal polyps Excision Sessile l Colon: colectomy l Rectum: staged with EUS or MRI • • Benign / Early malignant (T 1 No) : Transanal local excision or TEMS (may need further radical surgery) Other malignant : radical excision (APR /anterior resection)
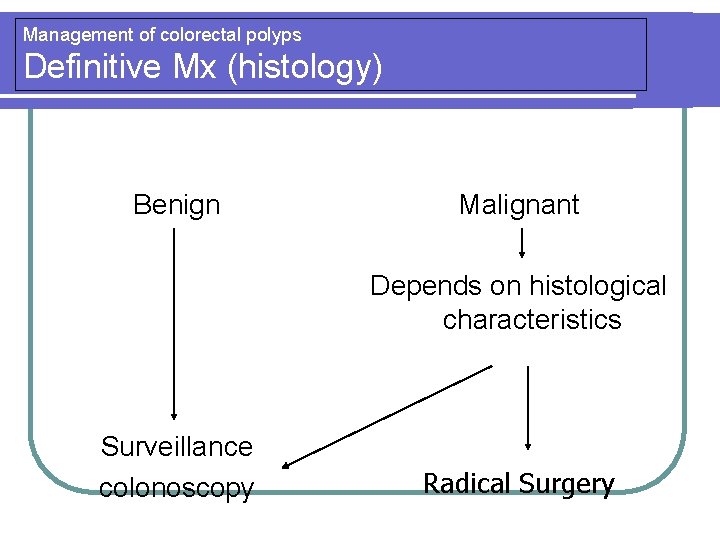
Management of colorectal polyps Definitive Mx (histology) Benign Malignant Depends on histological characteristics Surveillance colonoscopy Radical Surgery
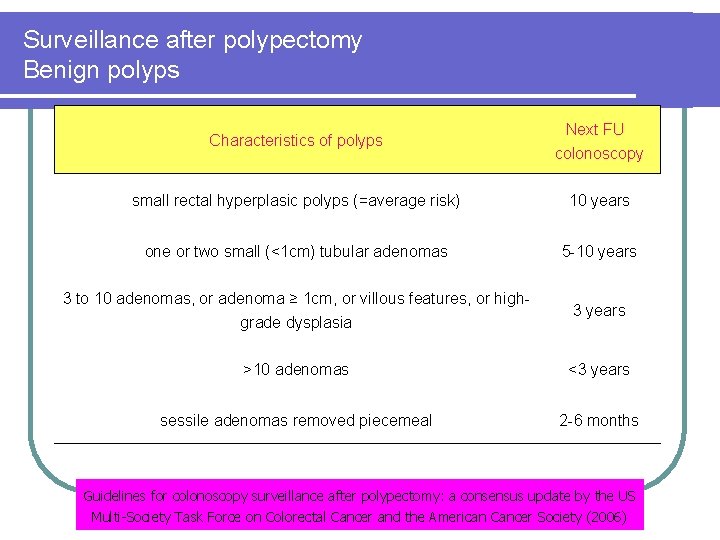
Surveillance after polypectomy Benign polyps Characteristics of polyps Next FU colonoscopy small rectal hyperplasic polyps (=average risk) 10 years one or two small (<1 cm) tubular adenomas 5 -10 years 3 to 10 adenomas, or adenoma ≥ 1 cm, or villous features, or highgrade dysplasia 3 years >10 adenomas <3 years sessile adenomas removed piecemeal 2 -6 months Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society (2006)
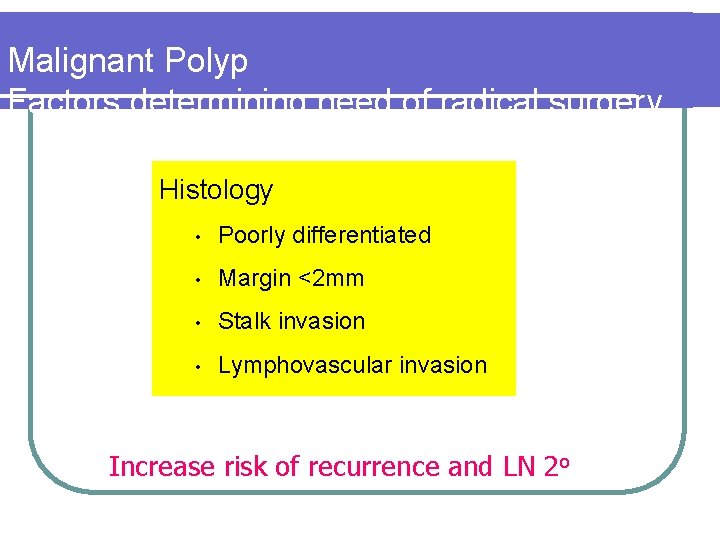
Malignant Polyp Factors determining need of radical surgery Histology • Poorly differentiated • Margin <2 mm • Stalk invasion • Lymphovascular invasion Increase risk of recurrence and LN 2 o
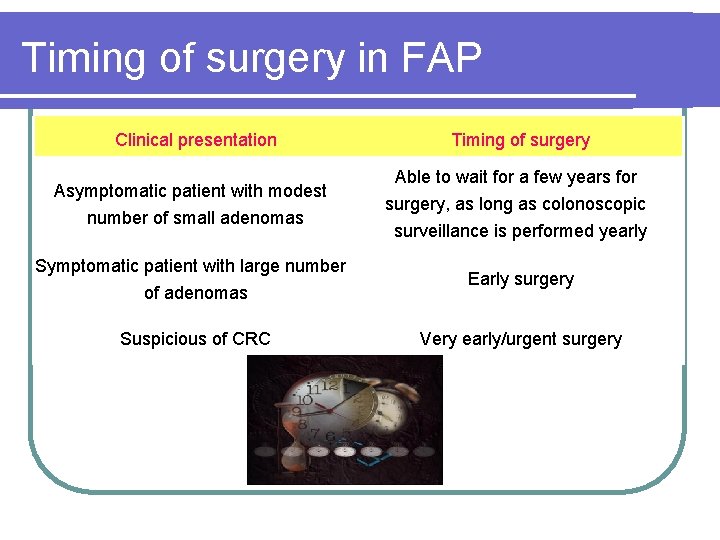
Timing of surgery in FAP Clinical presentation Asymptomatic patient with modest number of small adenomas Symptomatic patient with large number of adenomas Suspicious of CRC Timing of surgery Able to wait for a few years for surgery, as long as colonoscopic surveillance is performed yearly Early surgery Very early/urgent surgery
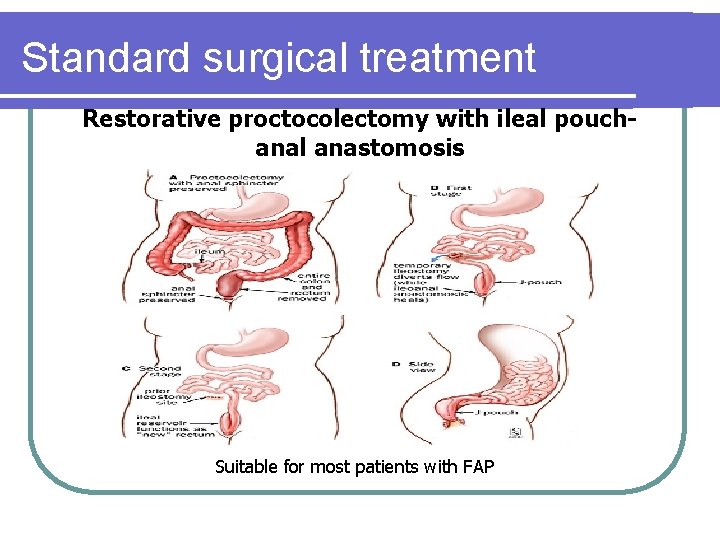
Standard surgical treatment Restorative proctocolectomy with ileal pouchanal anastomosis Suitable for most patients with FAP
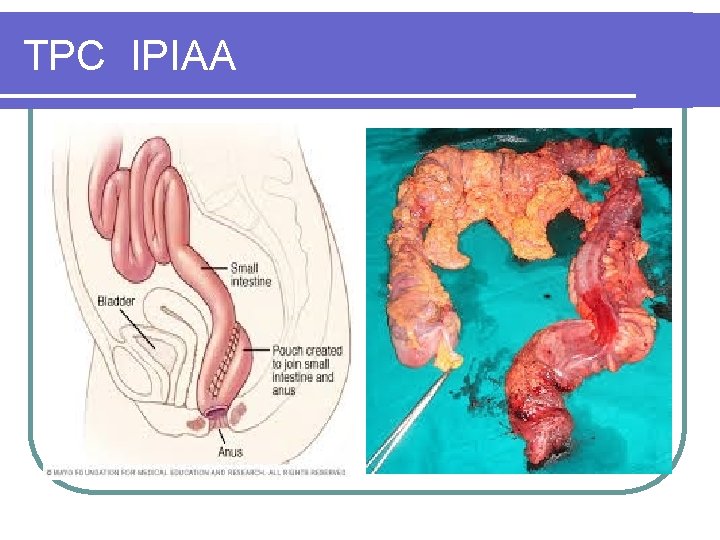
TPC IPIAA
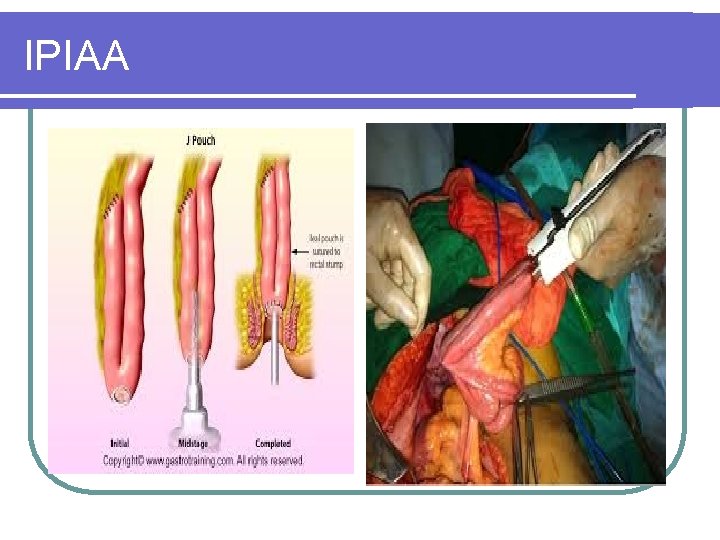
IPIAA
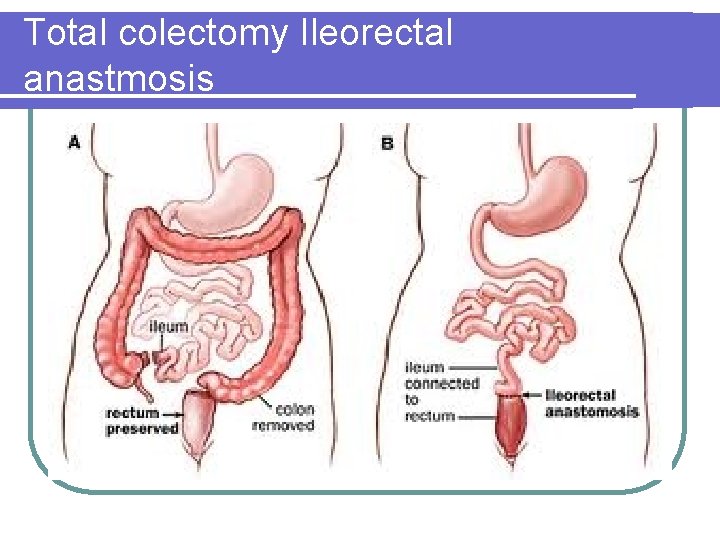
Total colectomy Ileorectal anastmosis
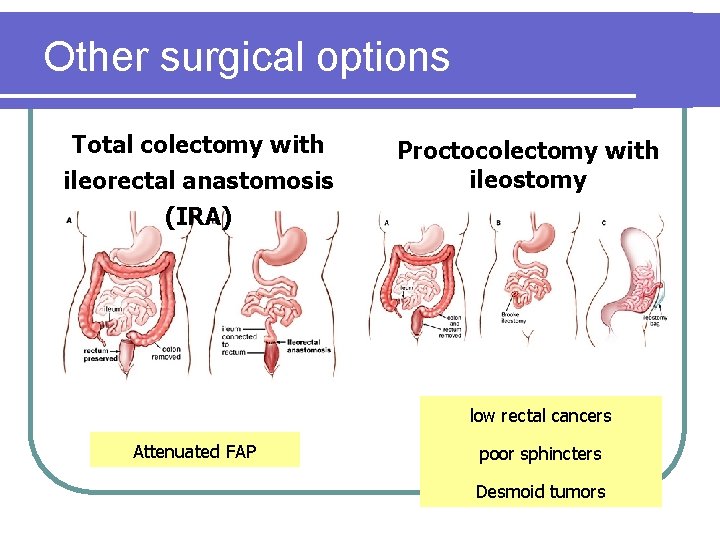
Other surgical options Total colectomy with ileorectal anastomosis (IRA) Proctocolectomy with ileostomy low rectal cancers Attenuated FAP poor sphincters Desmoid tumors
Medical treatment of FAP? l Sulindac (NSAID) and celecoxib (COX-2 inhibitor) shown to control and reduce the number of colorectal adenomas in FAP l Not definitive treatment l Temporizing treatment (eg when surgery needs to be delayed) l May control pouch and rectal polyposis after initial prophylactic surgery
Surgical treatment of HNPCC l Total colectomy with ileorectal anastomosis l Restorative proctocolectomy with ileal pouch-anal anastomosis l Segmental colectomy not recommended because of high rate of metachronous CRC l TAHBSO for endometrial cancer

- Slides: 63