Color Magnetism and Radioactivity Dr Stephen Crabtree Oct

Color, Magnetism, and Radioactivity Dr. Stephen Crabtree Oct. 1, 2018

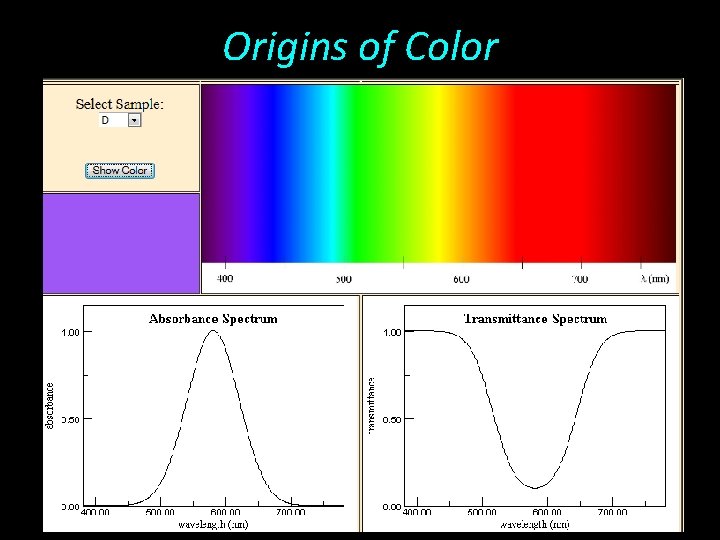
Origins of Color • White light hitting a mineral surface may be transmitted, scattered, reflected, refracted, or absorbed • Scattered and reflected light within the visible spectrum provides a mineral’s luster

Origins of Color • Colorless if all light is reflected • Colored if certain wavelengths of light are absorbed • Absorbed and transmitted waves can be quantitatively measured by scectroscopy
Origins of Color

Origins of Color • Three electron-based processes responsible for color: – Crystal Field Transitions – Molecular Orbital Transitions – Color Centers
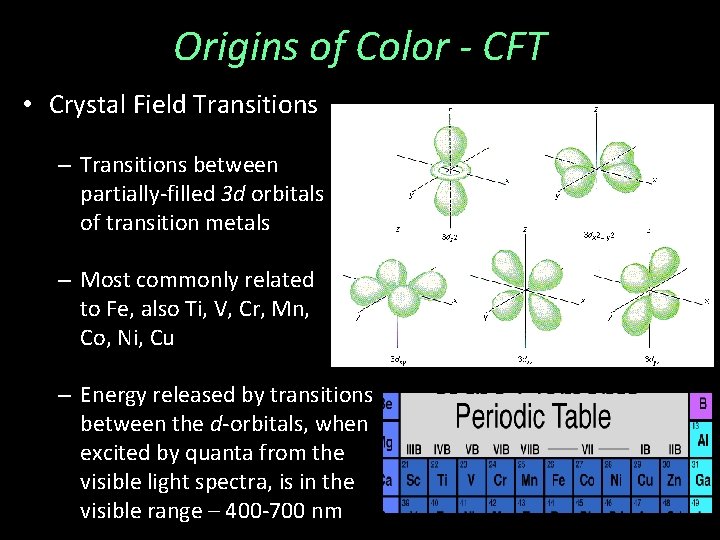
Origins of Color - CFT • Crystal Field Transitions – Transitions between partially-filled 3 d orbitals of transition metals – Most commonly related to Fe, also Ti, V, Cr, Mn, Co, Ni, Cu – Energy released by transitions between the d-orbitals, when excited by quanta from the visible light spectra, is in the visible range – 400 -700 nm
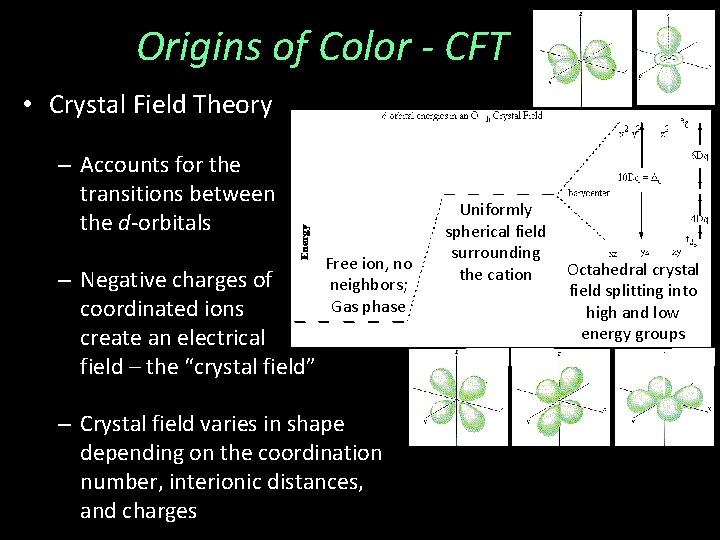
Origins of Color - CFT • Crystal Field Theory – Accounts for the transitions between the d-orbitals – Negative charges of coordinated ions create an electrical field – the “crystal field” Free ion, no neighbors; Gas phase – Crystal field varies in shape depending on the coordination number, interionic distances, and charges Uniformly spherical field surrounding the cation Octahedral crystal field splitting into high and low energy groups
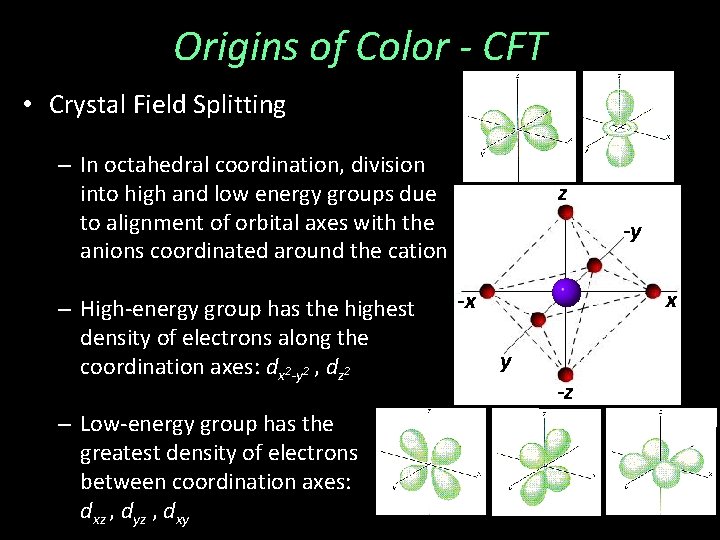
Origins of Color - CFT • Crystal Field Splitting – In octahedral coordination, division into high and low energy groups due to alignment of orbital axes with the anions coordinated around the cation – High-energy group has the highest density of electrons along the coordination axes: dx 2 -y 2 , dz 2 – Low-energy group has the greatest density of electrons between coordination axes: dxz , dyz , dxy z -y x -x y -z
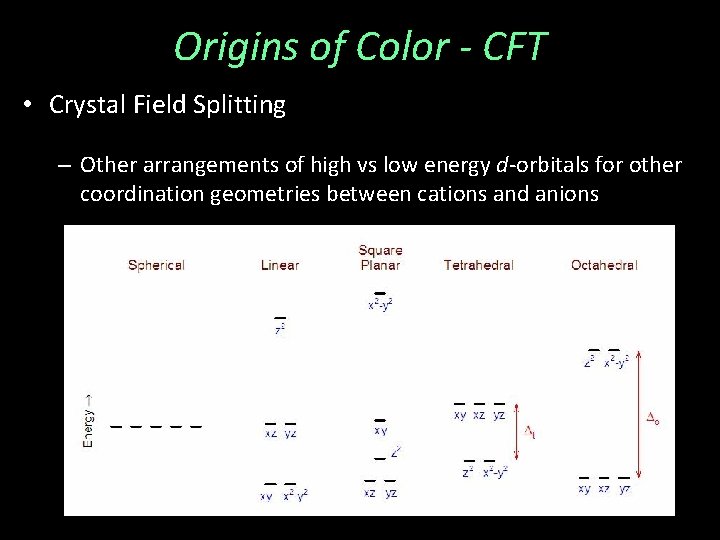
Origins of Color - CFT • Crystal Field Splitting – Other arrangements of high vs low energy d-orbitals for other coordination geometries between cations and anions
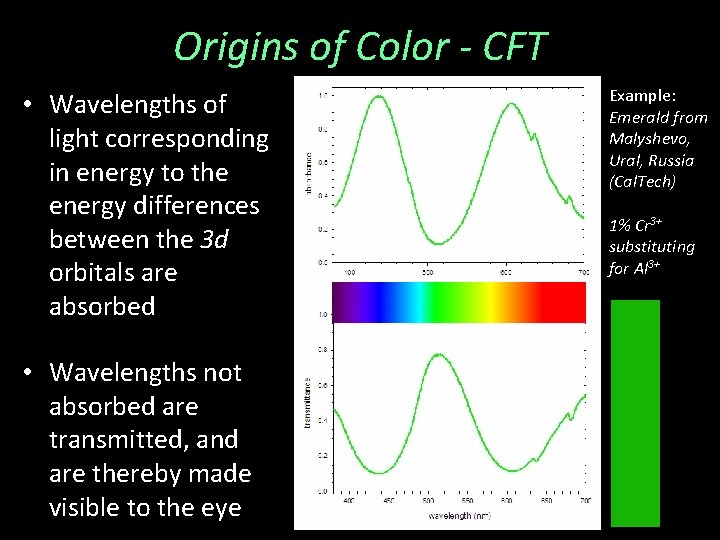
Origins of Color - CFT • Wavelengths of light corresponding in energy to the energy differences between the 3 d orbitals are absorbed • Wavelengths not absorbed are transmitted, and are thereby made visible to the eye Example: Emerald from Malyshevo, Ural, Russia (Cal. Tech) 1% Cr 3+ substituting for Al 3+
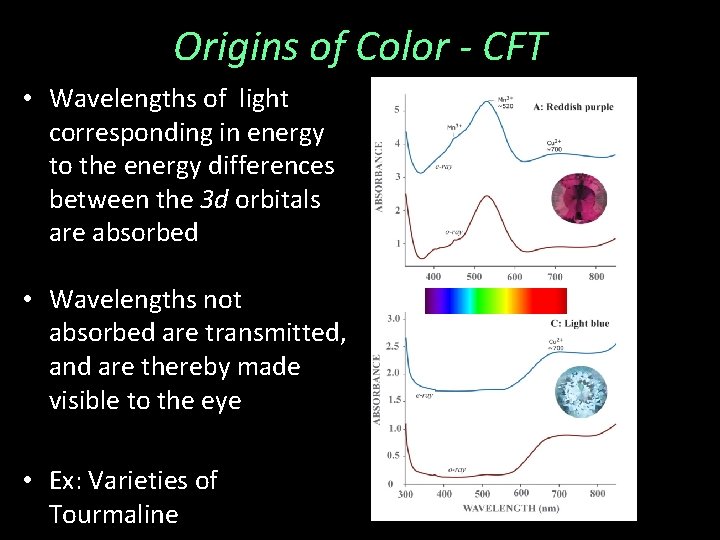
Origins of Color - CFT • Wavelengths of light corresponding in energy to the energy differences between the 3 d orbitals are absorbed • Wavelengths not absorbed are transmitted, and are thereby made visible to the eye • Ex: Varieties of Tourmaline
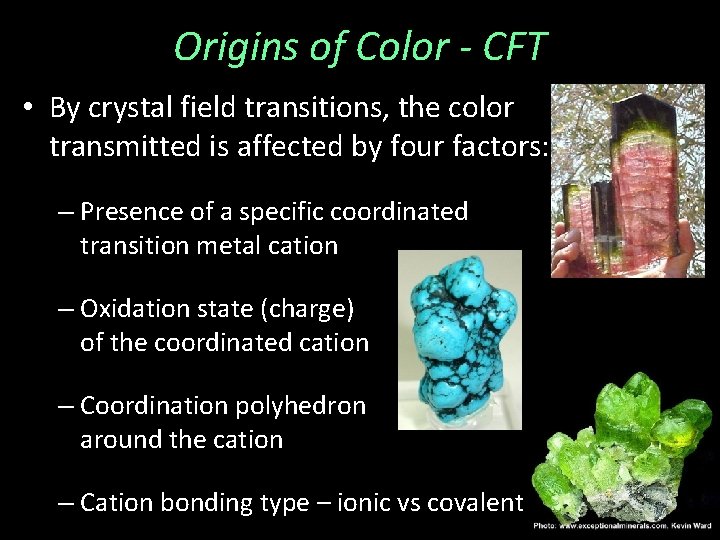
Origins of Color - CFT • By crystal field transitions, the color transmitted is affected by four factors: – Presence of a specific coordinated transition metal cation – Oxidation state (charge) of the coordinated cation – Coordination polyhedron around the cation – Cation bonding type – ionic vs covalent
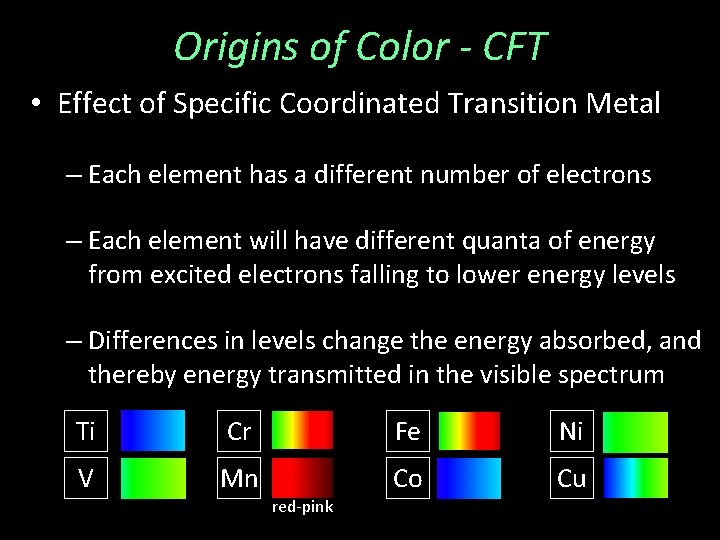
Origins of Color - CFT • Effect of Specific Coordinated Transition Metal – Each element has a different number of electrons – Each element will have different quanta of energy from excited electrons falling to lower energy levels – Differences in levels change the energy absorbed, and thereby energy transmitted in the visible spectrum Ti Cr Fe Ni V Mn Co Cu red-pink
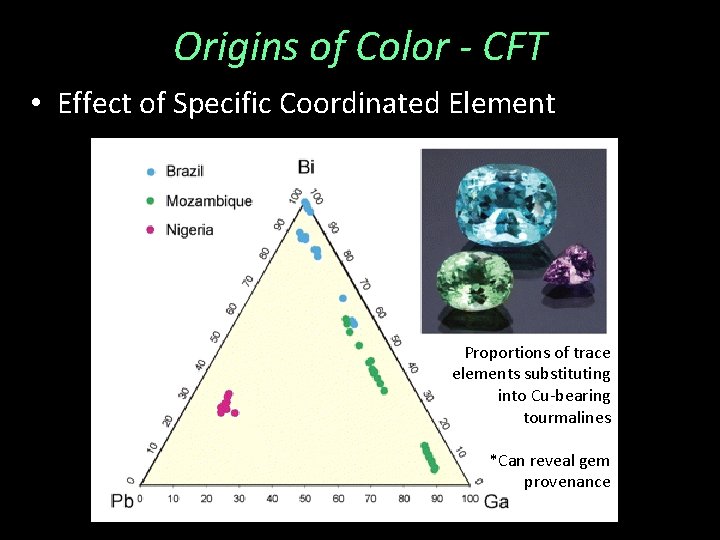
Origins of Color - CFT • Effect of Specific Coordinated Element Proportions of trace elements substituting into Cu-bearing tourmalines *Can reveal gem provenance

Origins of Color - CFT • Effect of Oxidation state of the Cation – Higher oxidation states may have skewed or weaker absorbance spectra – Example in iron-bearing minerals: Chrysoberyl Be. Al 2 O 4 with Fe 3+ Olivine (Fe. Mg)2 Si. O 4 with Fe 2+
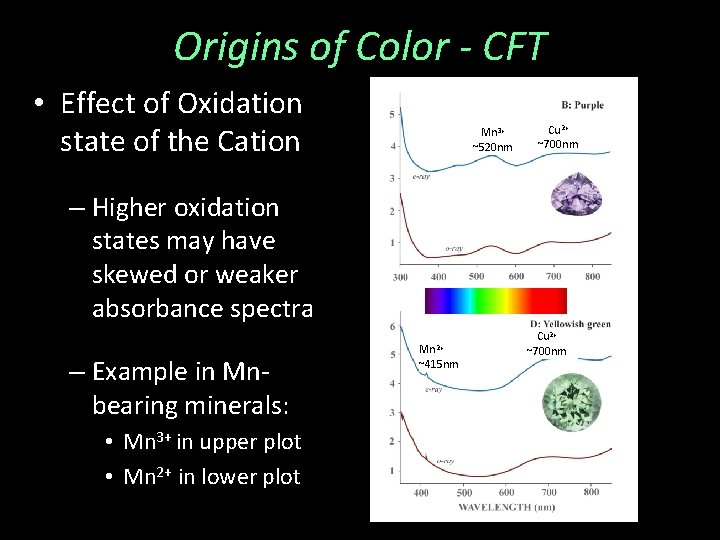
Origins of Color - CFT • Effect of Oxidation state of the Cation Mn 3+ ~520 nm Cu 2+ ~700 nm – Higher oxidation states may have skewed or weaker absorbance spectra – Example in Mnbearing minerals: • Mn 3+ in upper plot • Mn 2+ in lower plot Mn 2+ ~415 nm Cu 2+ ~700 nm

Origins of Color - CFT • Effect of Oxidation state of the Cation – Higher oxidation states may have skewed or weaker absorbance spectra Example of Mn-infused glass after UV exposure Manganese-tinted glass using Mn 3+ Manganese-tinted glass using Mn 2+
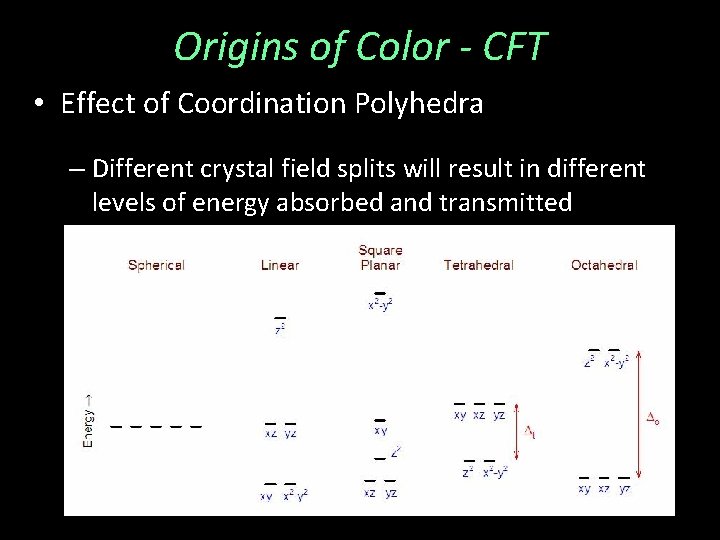
Origins of Color - CFT • Effect of Coordination Polyhedra – Different crystal field splits will result in different levels of energy absorbed and transmitted
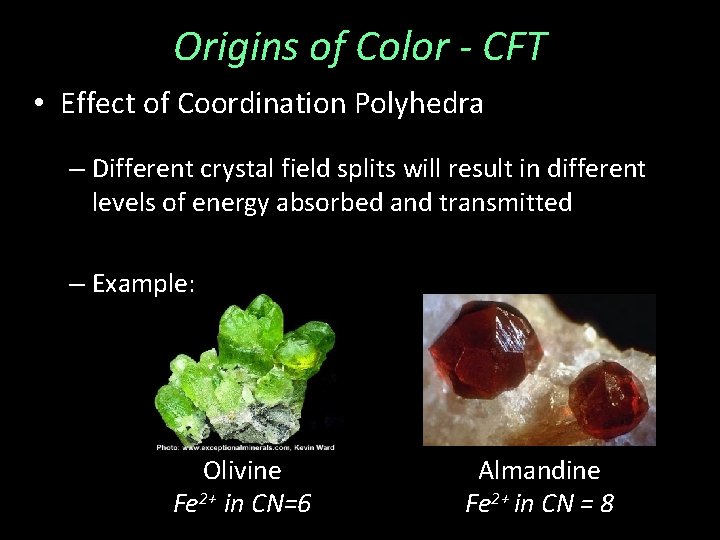
Origins of Color - CFT • Effect of Coordination Polyhedra – Different crystal field splits will result in different levels of energy absorbed and transmitted – Example: Olivine Fe 2+ in CN=6 Almandine Fe 2+ in CN = 8
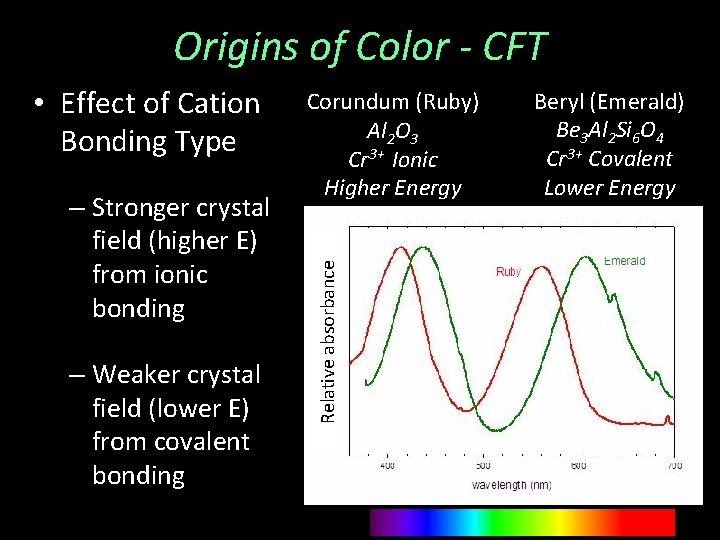
Origins of Color - CFT – Stronger crystal field (higher E) from ionic bonding – Weaker crystal field (lower E) from covalent bonding Corundum (Ruby) Al 2 O 3 Cr 3+ Ionic Higher Energy Relative absorbance • Effect of Cation Bonding Type Beryl (Emerald) Be 3 Al 2 Si 6 O 4 Cr 3+ Covalent Lower Energy
Origins of Color - CFT • By crystal field transitions, the color transmitted is affected by four factors: – Presence of a specific coordinated transition metal cation – Oxidation state (charge) of the coordinated cation – Coordination polyhedron the cation around – Cation bonding type – ionic vs covalent
Origins of Color - MOT • Molecular orbital transition – Also referred to as charge transfer transitions – Occur in minerals where valence electrons move between adjacent ions • Purely covalent – shared molecular orbitals – Crystal field theory does not apply here because the electrons are not localized around a singular cation
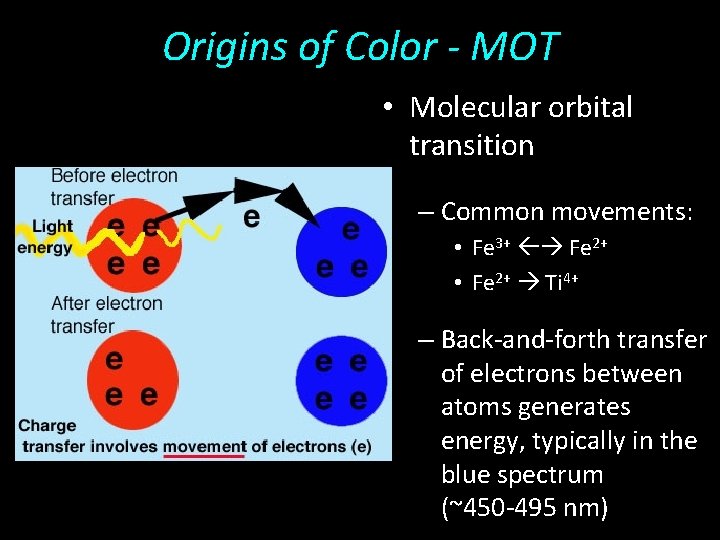
Origins of Color - MOT • Molecular orbital transition – Common movements: • Fe 3+ Fe 2+ • Fe 2+ Ti 4+ – Back-and-forth transfer of electrons between atoms generates energy, typically in the blue spectrum (~450 -495 nm)

Origins of Color - MOT • Molecular orbital transition Glaucophane Crocidolite Sapphire (Blue Corundum) Kyanite Cordierite – Common movements: • Fe 3+ Fe 2+ • Fe 2+ Ti 4+ – Back-and-forth transfer of electrons between atoms generates energy, typically in the blue spectrum (~450 -495 nm)
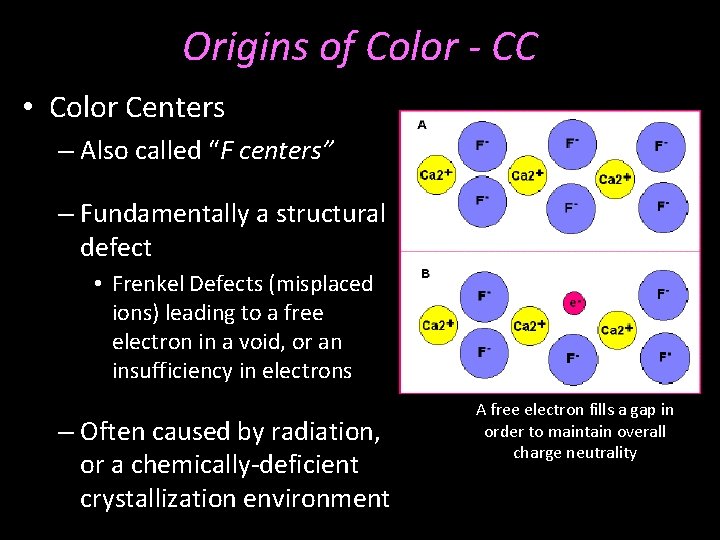
Origins of Color - CC • Color Centers – Also called “F centers” – Fundamentally a structural defect • Frenkel Defects (misplaced ions) leading to a free electron in a void, or an insufficiency in electrons – Often caused by radiation, or a chemically-deficient crystallization environment A free electron fills a gap in order to maintain overall charge neutrality
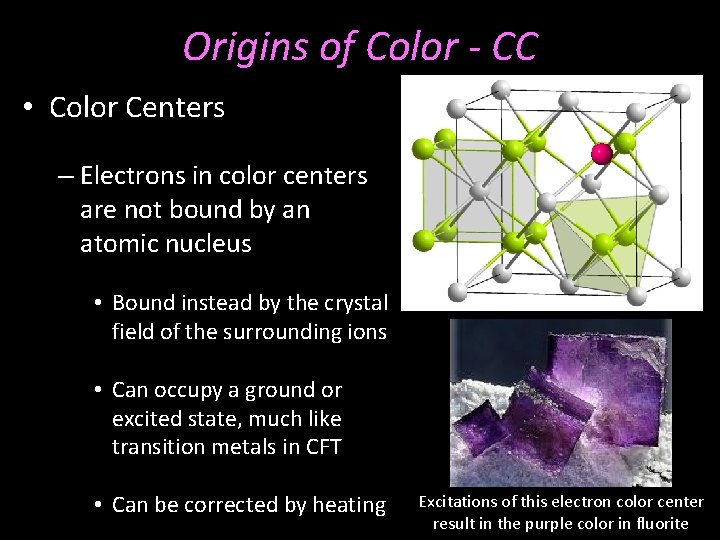
Origins of Color - CC • Color Centers – Electrons in color centers are not bound by an atomic nucleus • Bound instead by the crystal field of the surrounding ions • Can occupy a ground or excited state, much like transition metals in CFT • Can be corrected by heating Excitations of this electron color center result in the purple color in fluorite
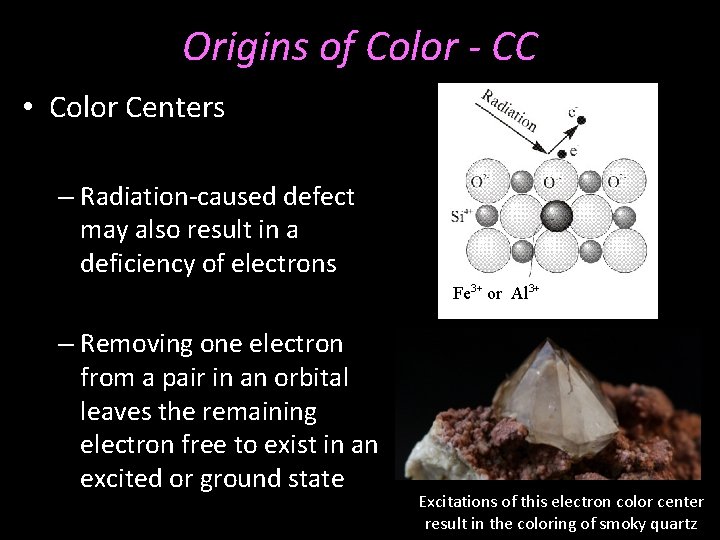
Origins of Color - CC • Color Centers – Radiation-caused defect may also result in a deficiency of electrons Fe 3+ or Al 3+ – Removing one electron from a pair in an orbital leaves the remaining electron free to exist in an excited or ground state Excitations of this electron color center result in the coloring of smoky quartz
Origins of Magnetism • Relates to the spin quantum number, s – Defines the spin of the electron in space s = +½ or -½ – Spinning electrons create magnetic fields while orbiting nuclei
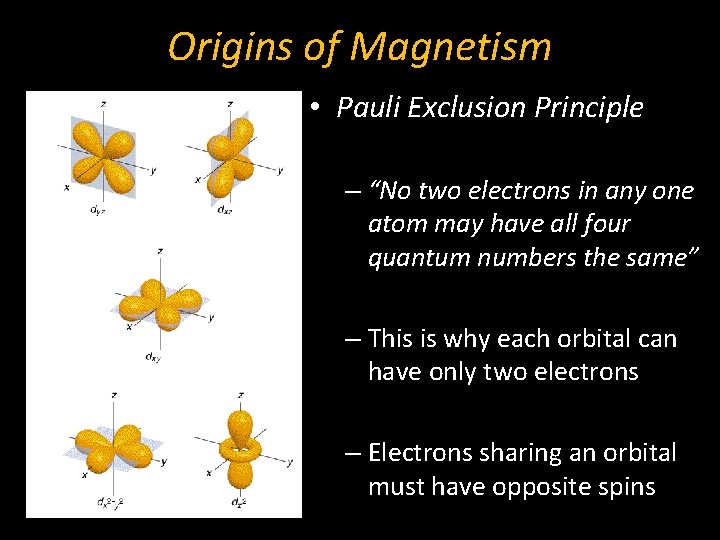
Origins of Magnetism • Pauli Exclusion Principle – “No two electrons in any one atom may have all four quantum numbers the same” – This is why each orbital can have only two electrons – Electrons sharing an orbital must have opposite spins
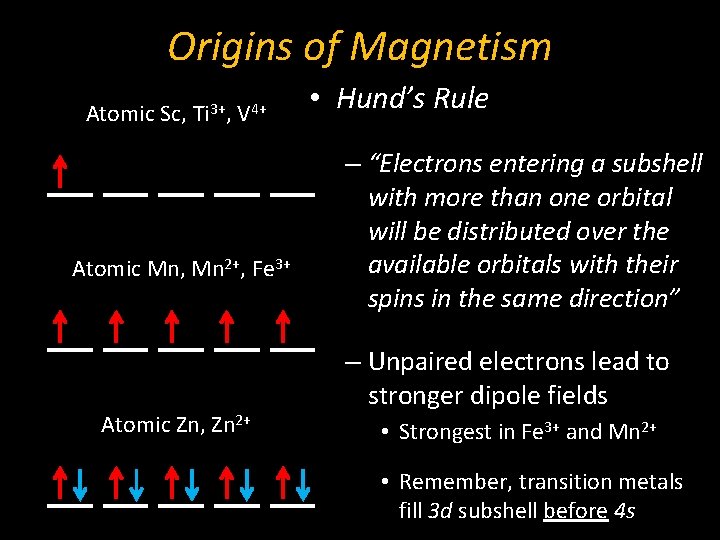
Origins of Magnetism Atomic Sc, Ti 3+, V 4+ Atomic Mn, Mn 2+, Fe 3+ Atomic Zn, Zn 2+ • Hund’s Rule – “Electrons entering a subshell with more than one orbital will be distributed over the available orbitals with their spins in the same direction” – Unpaired electrons lead to stronger dipole fields • Strongest in Fe 3+ and Mn 2+ • Remember, transition metals fill 3 d subshell before 4 s
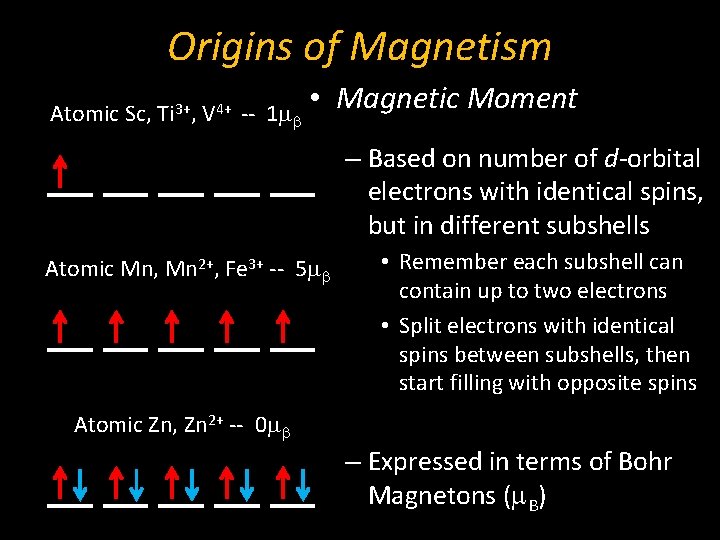
Origins of Magnetism Atomic Sc, Ti 3+, V 4+ -- 1 mb • Magnetic Moment – Based on number of d-orbital electrons with identical spins, but in different subshells Atomic Mn, Mn 2+, Fe 3+ -- 5 mb • Remember each subshell can contain up to two electrons • Split electrons with identical spins between subshells, then start filling with opposite spins Atomic Zn, Zn 2+ -- 0 mb – Expressed in terms of Bohr Magnetons (m. B)

Origins of Magnetism • Paramagnetism – Structure has a random arrangement of dipoles Examples: Olivine, Augite – When placed in a magnetic field, dipoles will align themselves, but only weakly – The magnetic attraction is not permenant
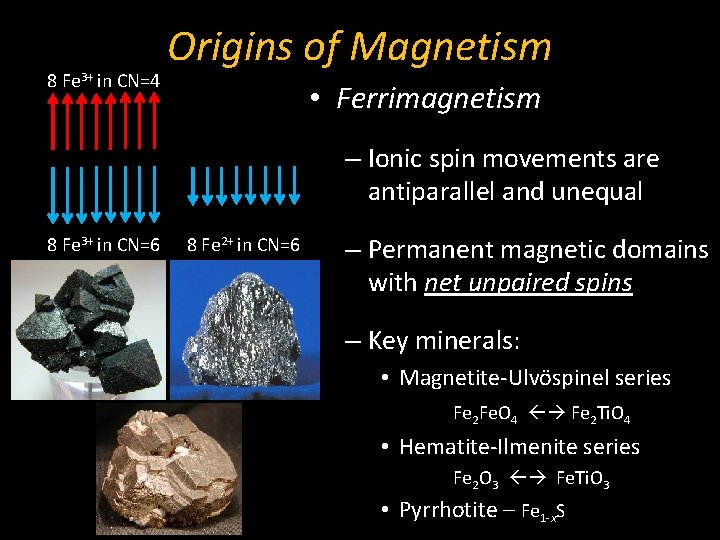
8 Fe 3+ in CN=4 Origins of Magnetism • Ferrimagnetism – Ionic spin movements are antiparallel and unequal 8 Fe 3+ in CN=6 8 Fe 2+ in CN=6 – Permanent magnetic domains with net unpaired spins – Key minerals: • Magnetite-Ulvöspinel series Fe 2 Fe. O 4 Fe 2 Ti. O 4 • Hematite-Ilmenite series Fe 2 O 3 Fe. Ti. O 3 • Pyrrhotite – Fe 1 -x. S

Origins of Radioactivity • Spontaneous decay of unstable nuclei, releasing radioactive energy Uraninite • Radioactive elements have decay constant, defining their half-lives • Give off gamma radiation (g) as a byproduct of radioactive decay as a nucleus changes from a high to low energy state Boltwoodite Monazite
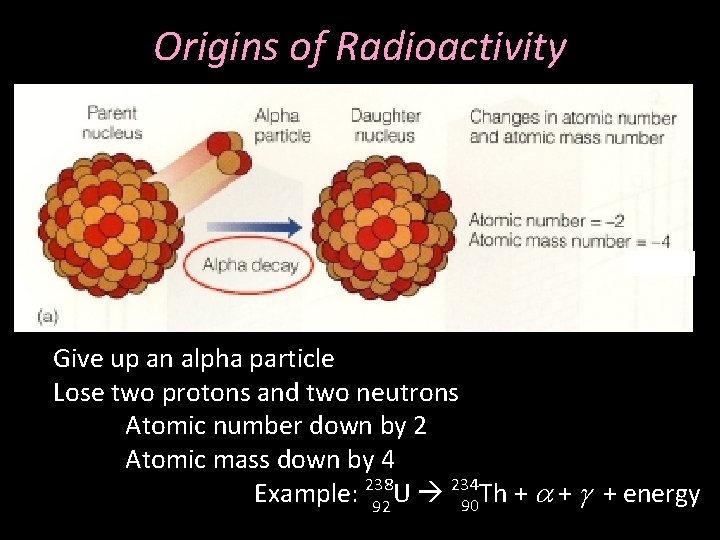
Origins of Radioactivity Give up an alpha particle Lose two protons and two neutrons Atomic number down by 2 Atomic mass down by 4 234 Example: 238 U 90 Th + a + g + energy 92
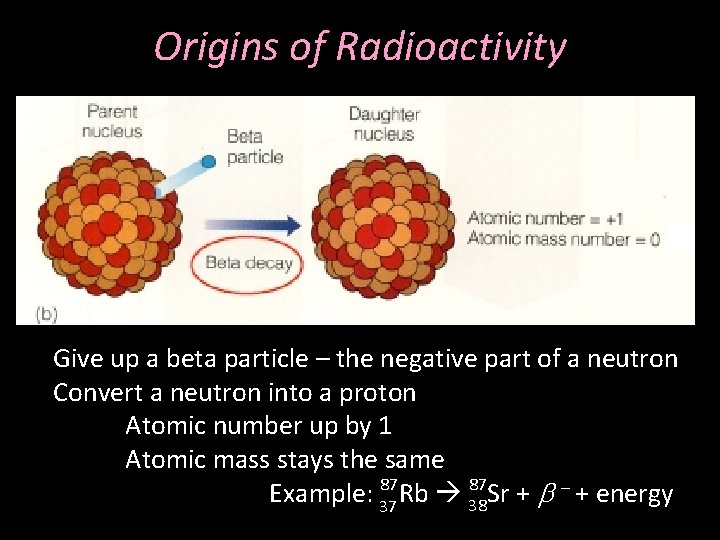
Origins of Radioactivity Give up a beta particle – the negative part of a neutron Convert a neutron into a proton Atomic number up by 1 Atomic mass stays the same 87 87 Example: 37 Rb 38 Sr + b - + energy
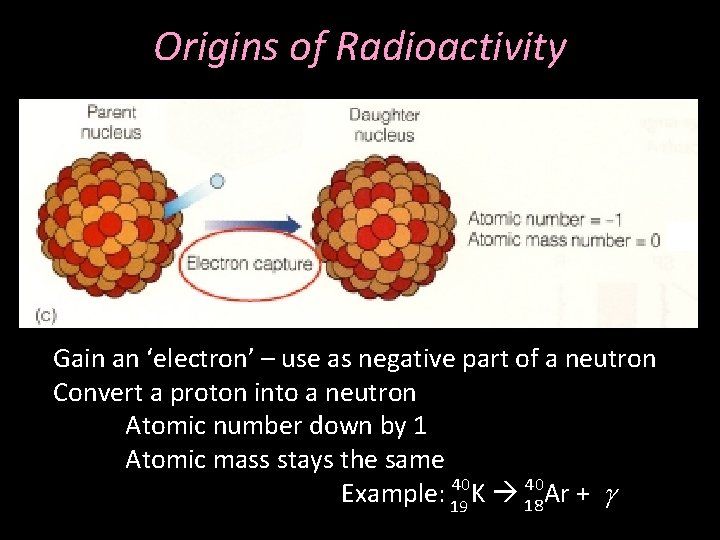
Origins of Radioactivity Gain an ‘electron’ – use as negative part of a neutron Convert a proton into a neutron Atomic number down by 1 Atomic mass stays the same 40 40 Example: 19 K 18 Ar + g

Origins of Radioactivity • Significant radioactive decay can be measured in minerals with abundant Uranium and Thorium Uraninite • Destructive effect of decay on crystal structures can be seen in metamict minerals Boltwoodite • Only detects radiation from the surface of a mineral, not interior Monazite
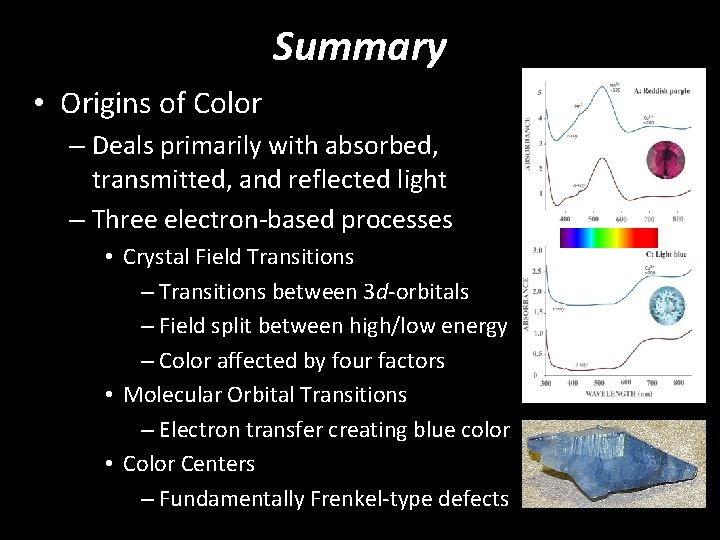
Summary • Origins of Color – Deals primarily with absorbed, transmitted, and reflected light – Three electron-based processes • Crystal Field Transitions – Transitions between 3 d-orbitals – Field split between high/low energy – Color affected by four factors • Molecular Orbital Transitions – Electron transfer creating blue color • Color Centers – Fundamentally Frenkel-type defects
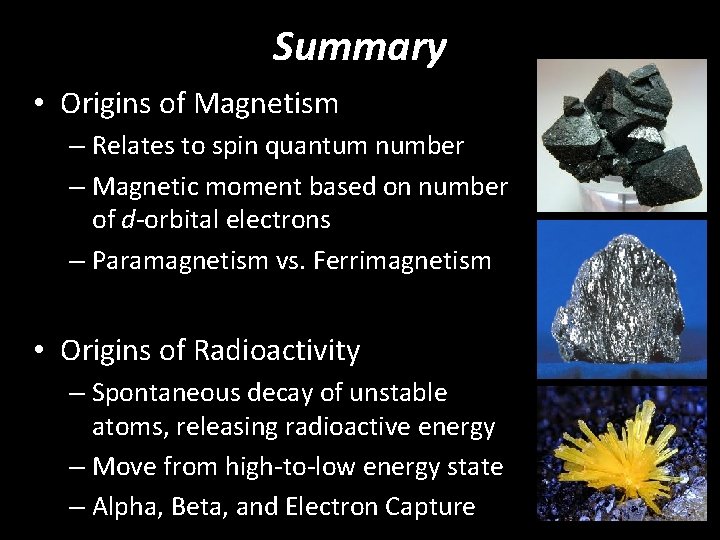
Summary • Origins of Magnetism – Relates to spin quantum number – Magnetic moment based on number of d-orbital electrons – Paramagnetism vs. Ferrimagnetism • Origins of Radioactivity – Spontaneous decay of unstable atoms, releasing radioactive energy – Move from high-to-low energy state – Alpha, Beta, and Electron Capture
After the Exams…The 2 nd ⅓ • External Symmetry Operations • Morphology Axes & Miller Indices • Crystal Classes & Space Groups
- Slides: 41