Colloids Colloids can be classified as particles in
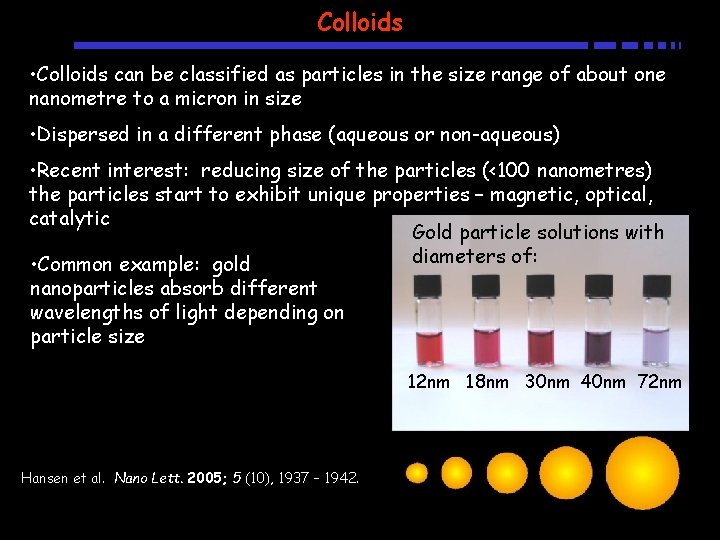
Colloids • Colloids can be classified as particles in the size range of about one nanometre to a micron in size • Dispersed in a different phase (aqueous or non-aqueous) • Recent interest: reducing size of the particles (<100 nanometres) the particles start to exhibit unique properties – magnetic, optical, catalytic Gold particle solutions with diameters of: • Common example: gold nanoparticles absorb different wavelengths of light depending on particle size 12 nm 18 nm 30 nm 40 nm 72 nm Hansen et al. Nano Lett. 2005; 5 (10), 1937 – 1942.
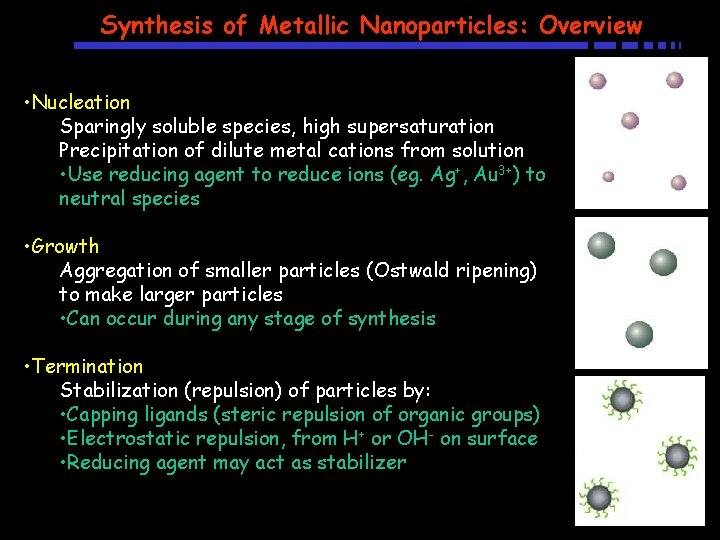
Synthesis of Metallic Nanoparticles: Overview • Nucleation Sparingly soluble species, high supersaturation Precipitation of dilute metal cations from solution • Use reducing agent to reduce ions (eg. Ag+, Au 3+) to neutral species • Growth Aggregation of smaller particles (Ostwald ripening) to make larger particles • Can occur during any stage of synthesis • Termination Stabilization (repulsion) of particles by: • Capping ligands (steric repulsion of organic groups) • Electrostatic repulsion, from H+ or OH- on surface • Reducing agent may act as stabilizer

Typical Nanoparticle Syntheses • Dilute solution of ions (<10 -4 M): Gold: tetrachloroauric acid (HAu. Cl 4) Silver: silver nitrate (Ag. NO 3) Platinum: hydrogen hexachloroplatinate (H 2 Pt. Cl 6) • Stabilizer is added, followed by reductant • Typical stabilizers: surfactants (SDS, CTAB, sodium 3 mercaptopropionate) • Typical reductants: Na. BH 4, N 2 H 4, sodium citrate • To ensure very small, homogeneous particles, reductant and stabilizer can be added together • Size can be varied by changing the Stabilizer/Metal (S/M) ratio Increasing the ratio decreases the avg. particle size Can achieve 3. 3 nm dia. Au particles with S/M = 5. 0* • Nanoparticles can remain stable in solution for several months *Kimizuka et al. Langmuir. 2000; 16, 5218 – 5220.
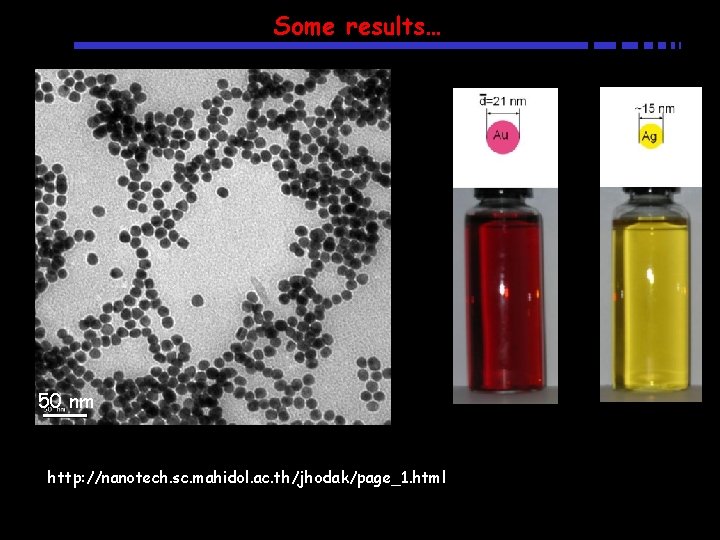
Some results… 50 nm http: //nanotech. sc. mahidol. ac. th/jhodak/page_1. html
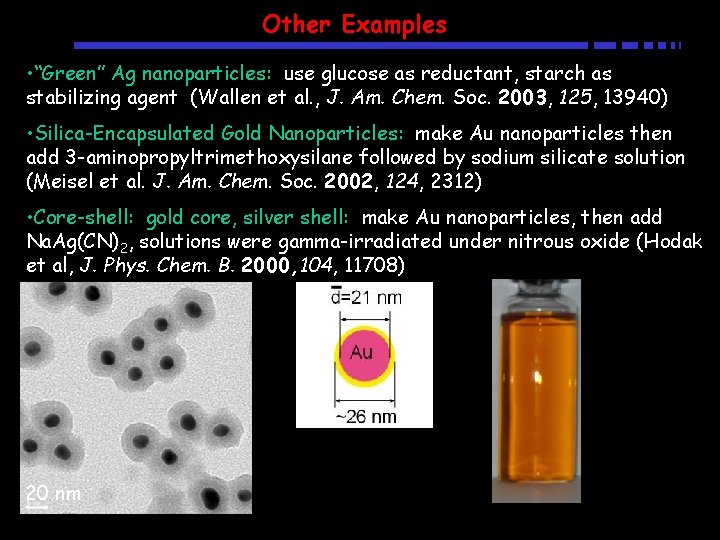
Other Examples • “Green” Ag nanoparticles: use glucose as reductant, starch as stabilizing agent (Wallen et al. , J. Am. Chem. Soc. 2003, 125, 13940) • Silica-Encapsulated Gold Nanoparticles: make Au nanoparticles then add 3 -aminopropyltrimethoxysilane followed by sodium silicate solution (Meisel et al. J. Am. Chem. Soc. 2002, 124, 2312) • Core-shell: gold core, silver shell: make Au nanoparticles, then add Na. Ag(CN)2, solutions were gamma-irradiated under nitrous oxide (Hodak et al, J. Phys. Chem. B. 2000, 104, 11708) 20 nm
- Slides: 5