Colloidal and surface phenomenal aspects of Ice cream















- Slides: 20

Colloidal and surface phenomenal aspects of Ice cream

History • Little is known • Introduced from Europe • Records indicate served by Governor Bladen of Maryland in 1700 • In 1832 a recipe and manufacturing methods were invented • First large scale ice cream plant established in 1851 in Baltimore

Design considerations • • • Flavor Texture Body Melt characteristics Color Inclusion of candies and fruit

Ingredients contributing to properties • • • Fat and water Emulsifiers Stabilizers Proteins Sweeteners Other elements

Fat and water • Fat – – Large molecule Triacylglyceride Nonpolar Vanderwaals • Water – Small molecule – Hydrogen dioxide – Polar

Emulsion – Mechanically dispersed – Low internal phase ratio – Low solubility (14 w/w) – Droplets. 1 -10 micro meter – Fat network • Large surface to volume ratio • Milk fat has wide melting pt around 40 C • This partial crystallinity adds to ability to network


Additional properties of fat • Produces characteristic smooth texture • Adds richness and sweetness in flavor • Aids in dissolving some flavors and vitamins

Emulsifiers • Emulsion stabilizers • Decrease interfacial tension from 15 -25 to less than 10 dynes/cm • Before freezing- decrease ability of fat to coalesce • During freezing-cause partial destabilization of lipid phase • During whipping- cause partial coalescence • When emulsifier conc. Increases fat penetrates air phase more • Results in stable air phase, stable fat network, smoothness in texture

Resulting benefits • • Decreased freezing time Increase minuteness of components Increase stiffness Increase uniformity of melting

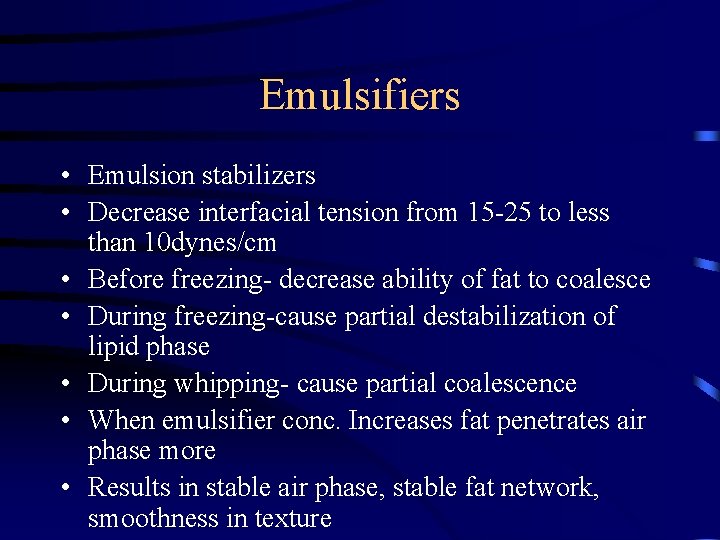
Types • Original: Lecithin • Found in egg yolks and soybeans • Phospholipids • Are modified for polarity and hydrophobicity

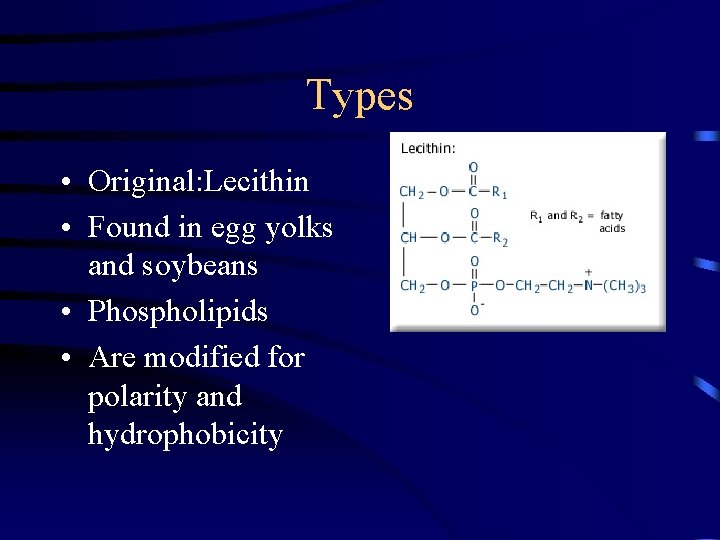
Types • • Poly sorbate Sorbitan ester Smaller in MW Produces low tension Very thin membrane Maximum fat destabilization Also drying agent-adsorbs some water

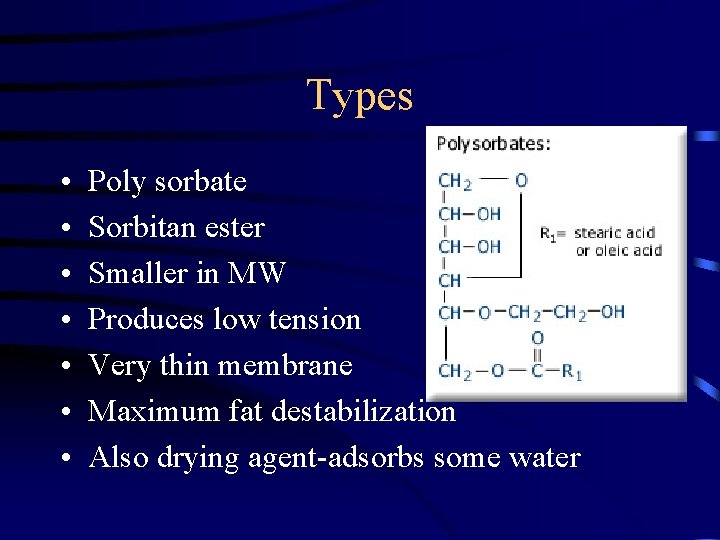
Types • Mono and Di glycerides • Derived from partial hydrolysis of fats and oils

Stabilizers • • • Effect ice/ water mixture Polysacharides – large hydration capability Increace viscocity decreacing diffusional abilities Stabilize foam phase Do not actively effect interfacial tension but decreace the avaliability of water indirectly effecting tension • Stabilize emulsion • Increace smoothness

Stabilizers cont. • Decrease size of ice crystals – Decrease diffusion and the total growth – Depresses freezing point as water is removed from solution maintaining water phase – Decreases “heat shock” through this mechanism • Increase stiffness of product • Decrease moisture migration out of product

Types • • • Carboxymethyl cellulose (CMC) Locust Bean Gum Carrageenan Guar gum Geltatin

Proteins • • • Large polymers Both hydrophobic and hydrophilic Extend on interface Create micelles Both stabilizers and emulsifiers increase protein concentration Increase viscosity Critical protein depletion before partial coalescence Increased hydrophobicity at interface yielding stability Decrease melting rates Increased shape retention

Types • Caseins – 80% total milk protein – Phosphoproteins precipitated at 4. 6 ph – Micelles – Preferentially diffuse out of lipid phase • Whey – Soluble at low ph – Globular

Sweeteners • Taste • Improve texture and palatability • Also depresses freezing point

Other ingredients and factors – Ions • effect destabilization, wetness • influence electric double layer and repulsion • citrate and phosphate increase protein aggregation and decrease coalescence • Ca and mg decrease aggregation and promote coalescence – Additives (nuts, candy etc. ) add crystal centers, also may effect moisture content – Phase volume – Temperature (freezing, mixing, packaging)