Collisions of Electrons with Atoms Quantum Physics Lesson








- Slides: 13

Collisions of Electrons with Atoms Quantum Physics Lesson 3

Today’s Objectives n Explain what is meant by the terms ionisation and excitation. n Define the electron volt (e. V) and be able to convert between Joules and e. V. n Describe what happens inside an atom when an electron becomes excited.

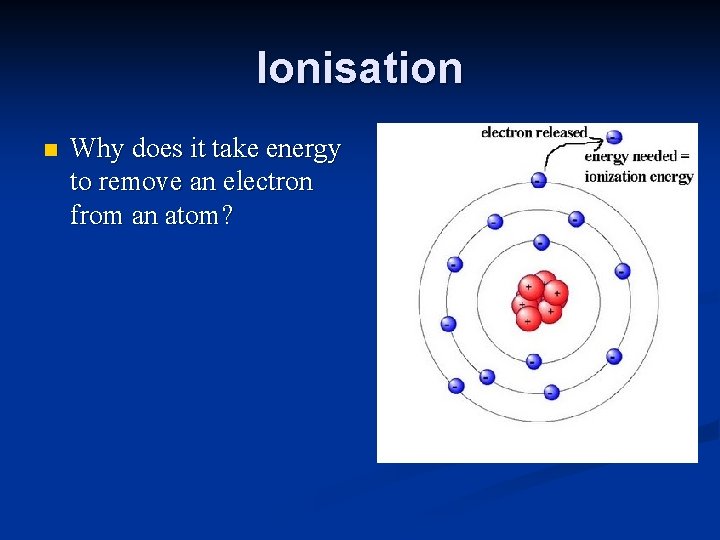
Ionisation What is an ion? An ion is a charged particle. n How is an ion formed from an atom? Electrons are either added or removed from the atom. n

Ionisation What happens if electrons are added to an atom? This makes the atom into a negative ion. n What happens if electrons are removed from an atom? This makes the atom into a positive ion. n

Ionisation n Why does it take energy to remove an electron from an atom?

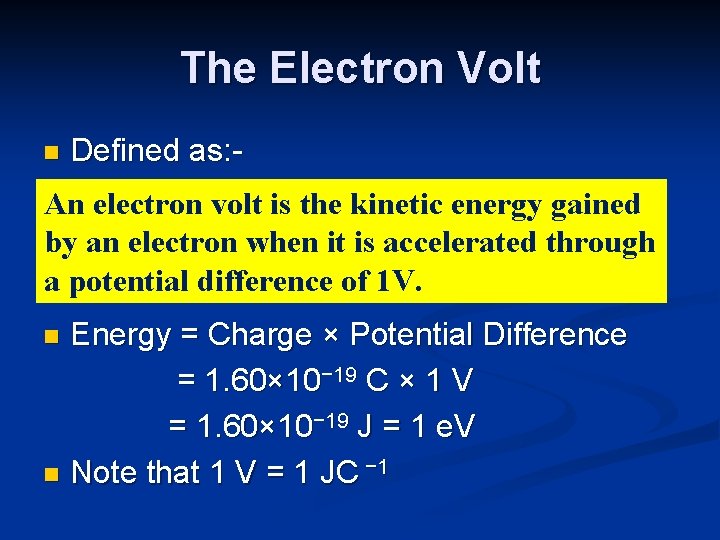
The Electron Volt n Defined as: - An electron volt is the kinetic energy gained by an electron when it is accelerated through a potential difference of 1 V. Energy = Charge × Potential Difference = 1. 60× 10− 19 C × 1 V = 1. 60× 10− 19 J = 1 e. V n Note that 1 V = 1 JC − 1 n

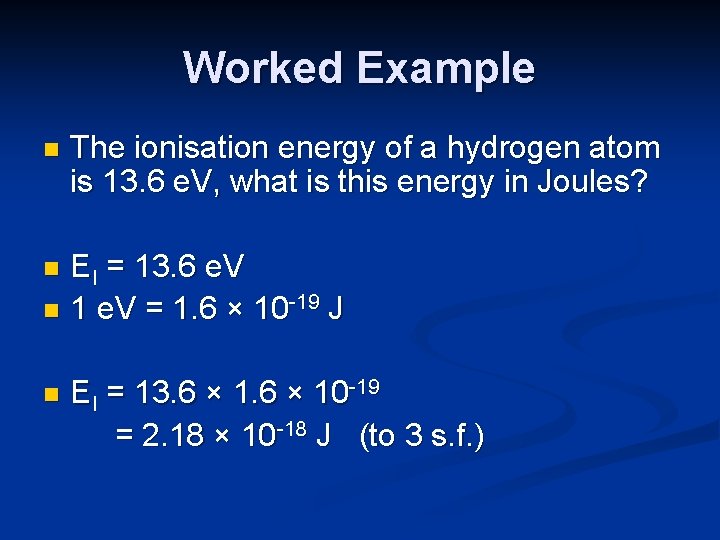
Worked Example n The ionisation energy of a hydrogen atom is 13. 6 e. V, what is this energy in Joules? EI = 13. 6 e. V n 1 e. V = 1. 6 × 10 -19 J n n EI = 13. 6 × 10 -19 = 2. 18 × 10 -18 J (to 3 s. f. )

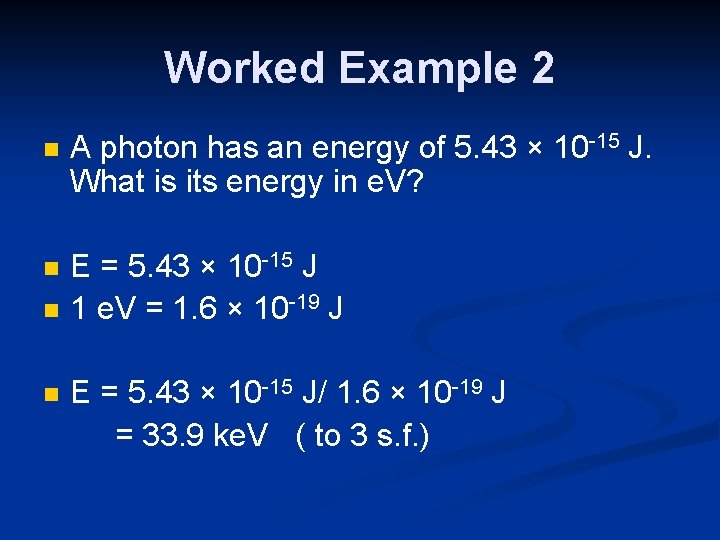
Worked Example 2 n A photon has an energy of 5. 43 × 10 -15 J. What is its energy in e. V? n E = 5. 43 × 10 -15 J 1 e. V = 1. 6 × 10 -19 J n n E = 5. 43 × 10 -15 J/ 1. 6 × 10 -19 J = 33. 9 ke. V ( to 3 s. f. )

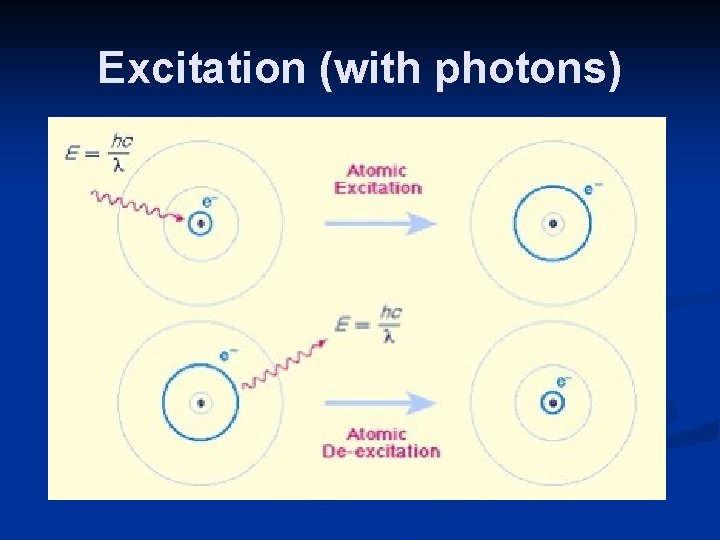
Excitation n Gas atoms can absorb energy from colliding electrons without being ionised excitation. n This happens at specific energies called excitation energies. n When excitation occurs the colliding electron makes an electron inside the atom move from an inner shell to an outer shell.

Excitation (with photons)

Excitation n If the colliding electron doesn’t have enough kinetic energy it is just deflected with no loss of kinetic energy. n The excitation is always less than the ionisation energy because the electron is not removed from the atom.

Definitions Ionisation When an atom loses an orbiting electron (and becomes charged) from exam. An electron is removed from an atom (making it a positive ion). from exam. Excitation An electron is raised to a higher energy level but remains within the atom.

Summary n Ionisation an electron is removed from the atom. n Excitation an atomic electron is moved from an inner shell to an outer shell. n Electron volt is the kinetic energy gained by an electron when it is accelerated through a potential difference of 1 V.