Collision Theory Unit 3 Reaction Rate Collision Theory








- Slides: 10

Collision Theory Unit 3 – Reaction Rate

Collision Theory Chemical reactions occur at different rates, some faster than others. The instantaneous rate of a reaction changes throughout the reaction, it is quick at the very beginning and slows down as the reaction progresses. What causes a chemical reaction? Why is the reaction rate more rapid at the beginning? Why do all the particles of reactants not change at the same time? What is the mechanism that changes them?

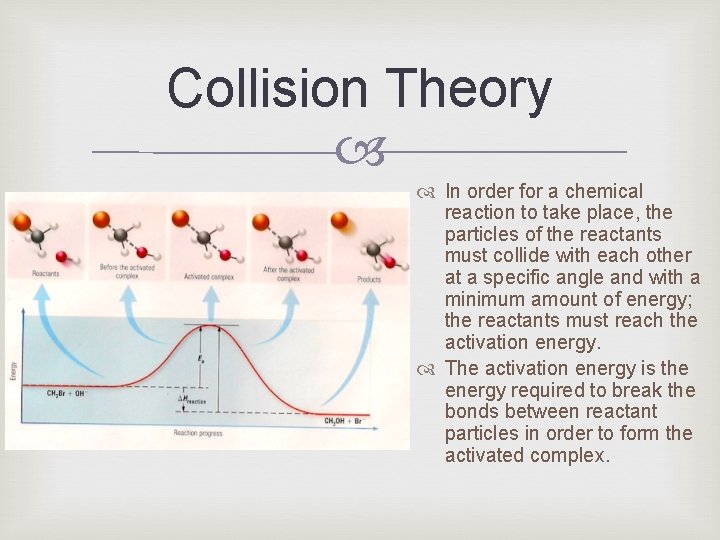
Collision Theory In order for a chemical reaction to take place, the particles of the reactants must collide with each other at a specific angle and with a minimum amount of energy; the reactants must reach the activation energy. The activation energy is the energy required to break the bonds between reactant particles in order to form the activated complex.

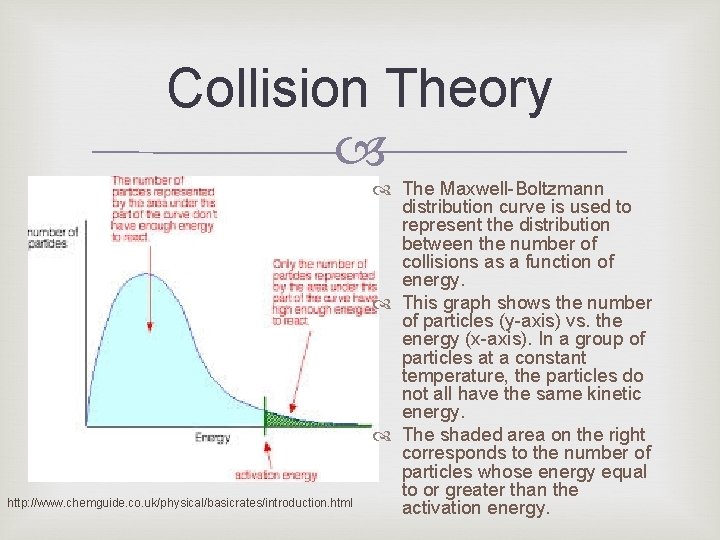
Collision Theory http: //www. chemguide. co. uk/physical/basicrates/introduction. html The Maxwell-Boltzmann distribution curve is used to represent the distribution between the number of collisions as a function of energy. This graph shows the number of particles (y-axis) vs. the energy (x-axis). In a group of particles at a constant temperature, the particles do not all have the same kinetic energy. The shaded area on the right corresponds to the number of particles whose energy equal to or greater than the activation energy.

Elastic Collision An elastic collision is a collision that does not result in a chemical reaction. The energy of the particles is lower than the activation energy and/or the particles are colliding on the wrong orientation. When the energy of the reactant particles is lower than the activation energy and/or the particles are colliding in the wrong orientation, the particles bounce without being transformed or losing energy.

Inelastic Collision An inelastic collision is a collision that results in a chemical reaction. In order for an inelastic reaction to occur the molecule must have the correct orientation and enough energy to be equal to or greater than the activation energy. The number of inelastic collisions determines the rate of a reaction. The more inelastic collisions there are in a given period of time, the faster the reaction rate.

Collision Theory What parameters must be changed in order to increase the number of inelastic collisions in a reaction? Increase the total number of particles. Increase the average kinetic energy. Decrease the activation energy required.

Collision Theory Remember that some chemical reactions are complex and occur in several steps. Each step is an elementary reaction that sets in motion a single transformation at the molecular level. At the end of each step, the products formed become the reactants for the subsequent step. This occurs over and over again until the final product is formed. The sum of the elementary reactions is the overall reaction (Hess’s law).

Collision Theory The products that are formed in between the initial reactants and the final products are intermediates. Since each step is an elementary reaction, the formation of the intermediates depends on the energy and the orientation of the collisions between the reactants. The energy level of the activated complex determines the rate of each step in the overall reaction.

Rate-Determining Step The step that is the slowest is called the ratedetermining step. The rate-determining step is the step that limits the overall rate and is therefore the one whose activated complex has the highest energy. It takes a lot of energy to reach the activated complex with the highest energy regardless of its activation energy.