Collision Theory Collision Theory What is necessary for





- Slides: 17

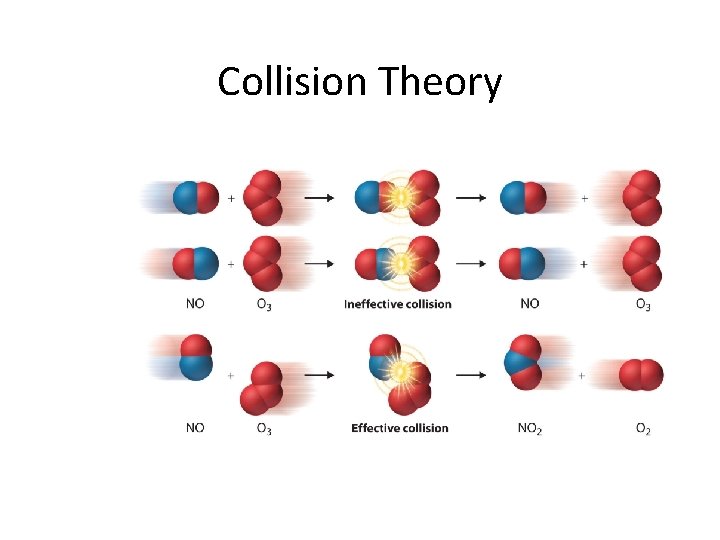
Collision Theory

Collision Theory • What is necessary for a chemical reaction to occur? • Collision theory – theory that a reaction occurs when an effective collision occurs between reactants (atoms, molecules or ions) • What makes a collision effective?

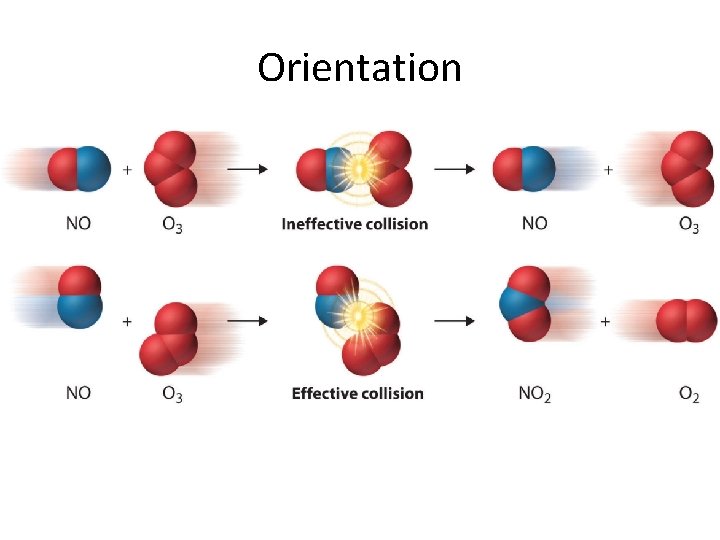
Effective Collisions 1. the orientation of the reactants (the collision geometry) must be favourable 2. the collision must occur with sufficient energy

Orientation

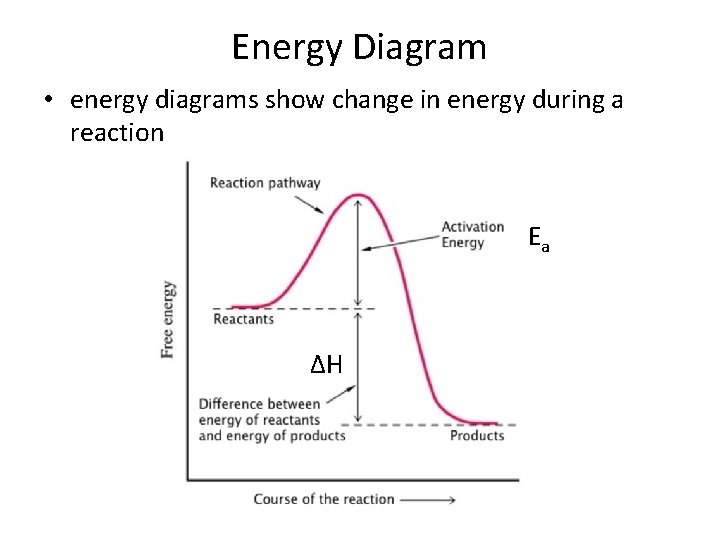
Energy • Activation energy (Ea) – the minimum collision energy required for the reaction to take place • This energy is needed first to break the bonds in the reactants and then to form new bonds in the products

Energy Diagram • energy diagrams show change in energy during a reaction Ea ΔH

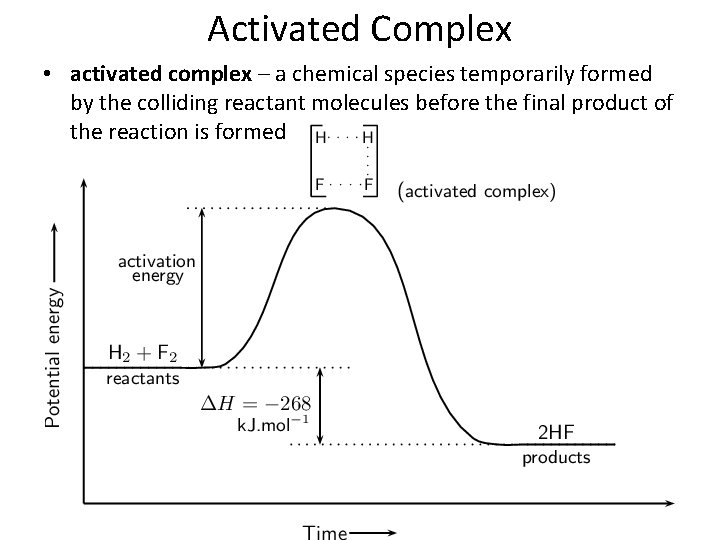
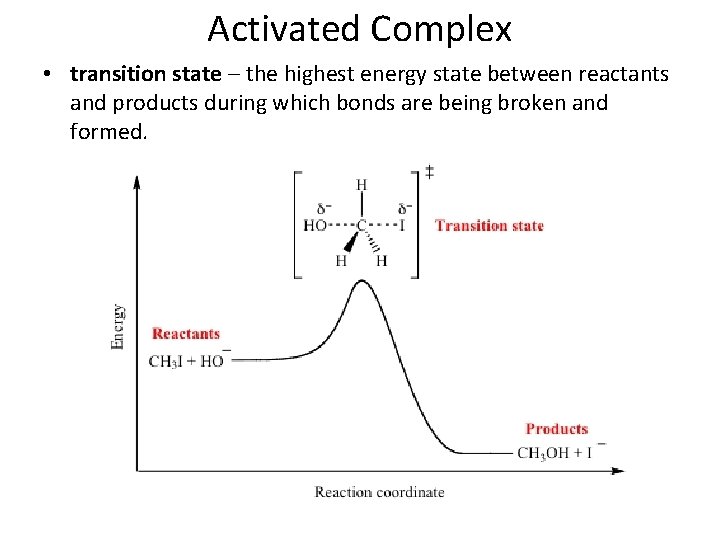
Activated Complex • activated complex – a chemical species temporarily formed by the colliding reactant molecules before the final product of the reaction is formed

Activated Complex • transition state – the highest energy state between reactants and products during which bonds are being broken and formed.

Ea • How will activation energy affect the rate of a reaction? • The higher the activation energy the fewer the effective collisions and the slower the reaction

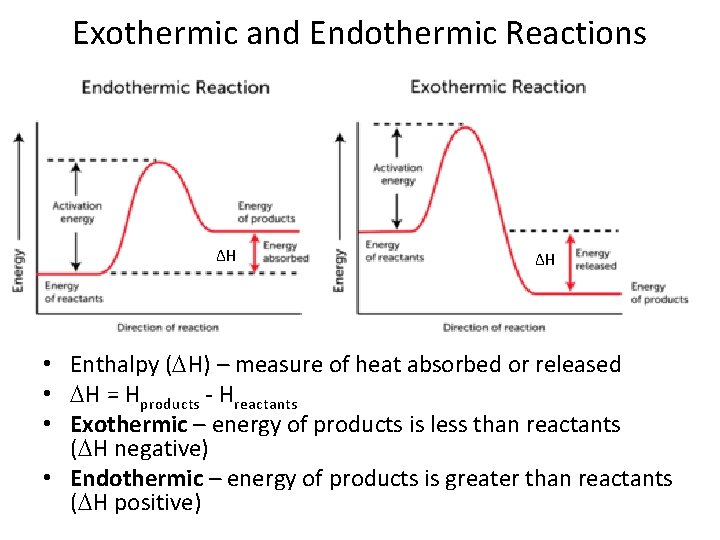
Exothermic and Endothermic Reactions ΔH ΔH • Enthalpy ( H) – measure of heat absorbed or released • H = Hproducts - Hreactants • Exothermic – energy of products is less than reactants ( H negative) • Endothermic – energy of products is greater than reactants ( H positive)

Factors Affecting Reaction Rate 1. 2. 3. 4. 5. Nature of reactants Temperature Concentration Surface Area Catalysts

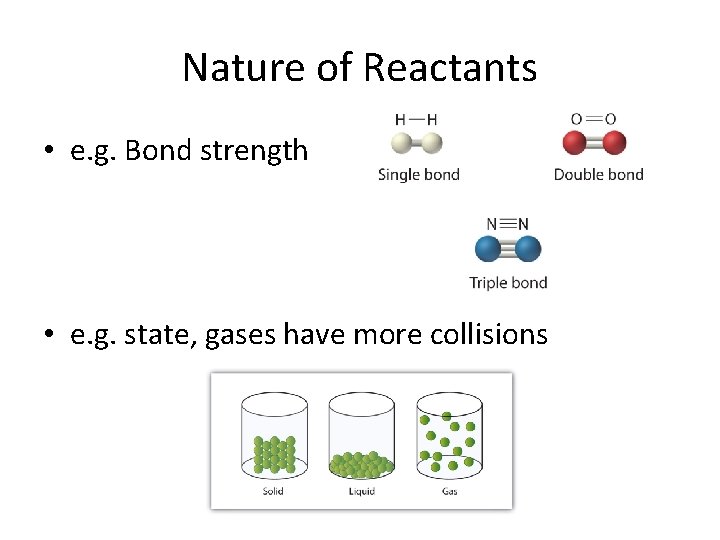
Nature of Reactants • e. g. Bond strength • e. g. state, gases have more collisions

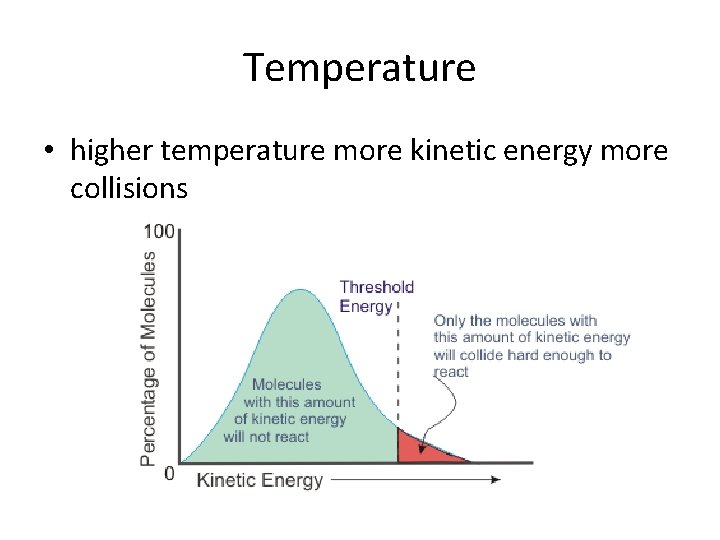
Temperature • higher temperature more kinetic energy more collisions

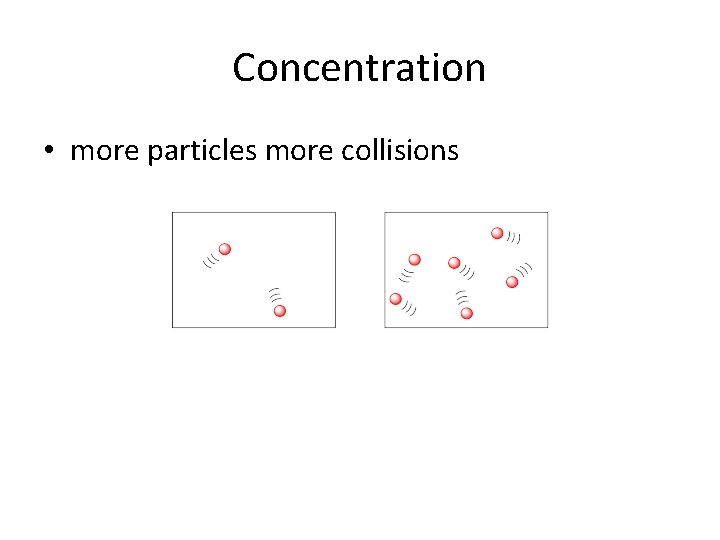
Concentration • more particles more collisions

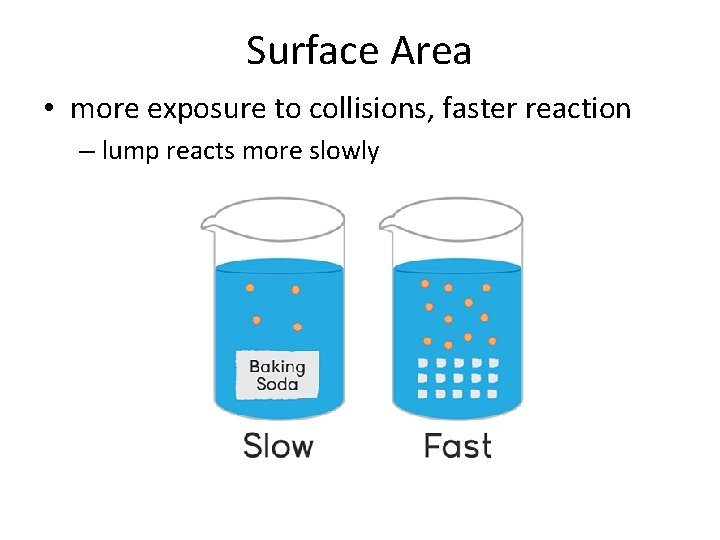
Surface Area • more exposure to collisions, faster reaction – lump reacts more slowly

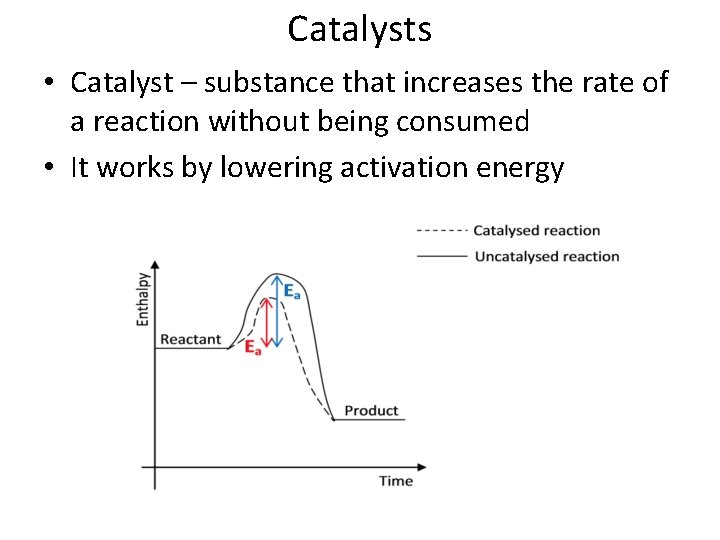
Catalysts • Catalyst – substance that increases the rate of a reaction without being consumed • It works by lowering activation energy

Inhibitor • inhibitor – slows a reaction