COLLISION FREQUENCY The number of collision which takes
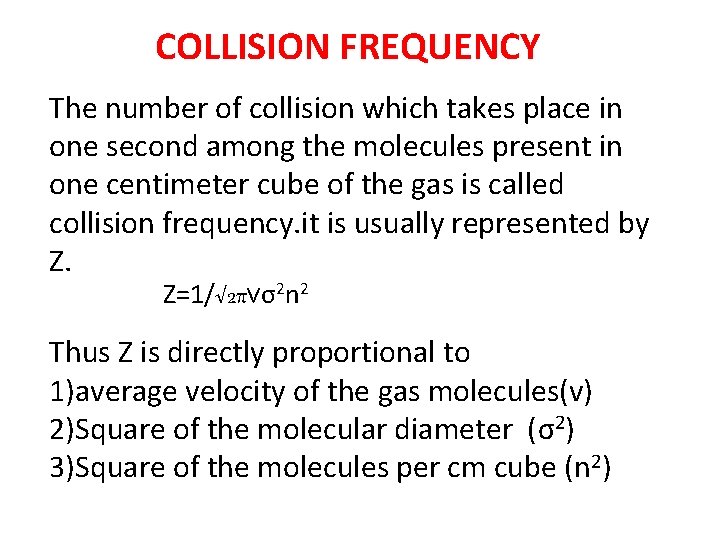
COLLISION FREQUENCY The number of collision which takes place in one second among the molecules present in one centimeter cube of the gas is called collision frequency. it is usually represented by Z. Z=1/√ 2πνσ2 n 2 Thus Z is directly proportional to 1)average velocity of the gas molecules(v) 2)Square of the molecular diameter (σ2) 3)Square of the molecules per cm cube (n 2)
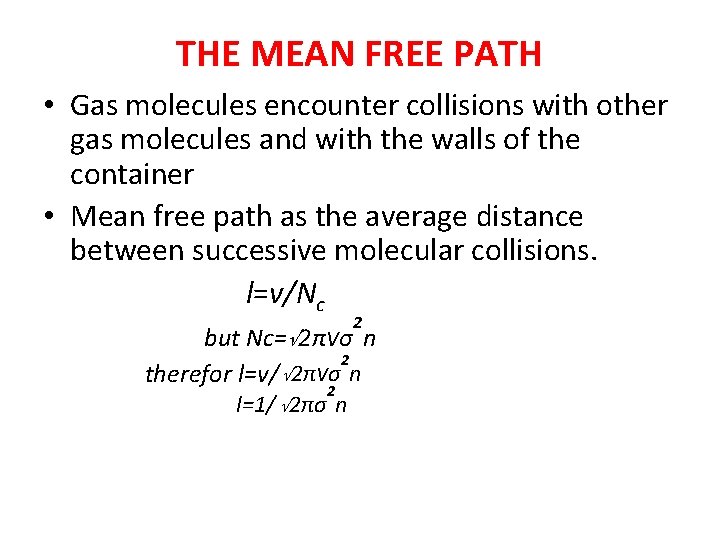
THE MEAN FREE PATH • Gas molecules encounter collisions with other gas molecules and with the walls of the container • Mean free path as the average distance between successive molecular collisions. l=v/Nc 2 but Nc=√ 2πνσ n 2 therefor l=v/ √ 2πνσ n 2 l=1/ √ 2πσ n
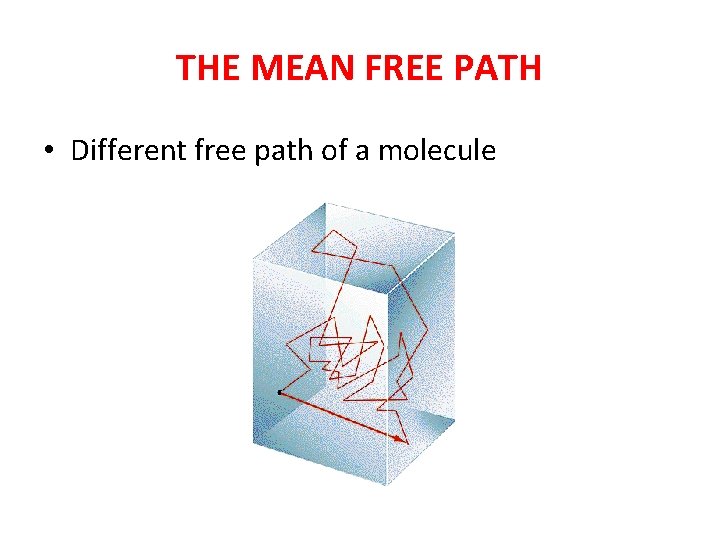
THE MEAN FREE PATH • Different free path of a molecule
IDEAL AND REAL GASES IDEAL GAS: - A gas which obeys the gas equation (PV=n. RT) under all condition of temperature and pressure is called an ideal gas. The concept of ideal gas is only hypothetical. for example: hydrogen, oxygen, nitrogen REAL GAS: A gas which obeys the gas laws fairly well under low pressure or high temperature. The concept of real gases is real. for example: carbon dioxide, sulpur dioxide , ammonia

DEVIATION FROM THE GAS LAWS AND EXPLAINATION IN TERM OF COMPRESSIBILITY FACTOR AND BOYLE TEMPERATURE • The Effect of temperature and pressure on the behaviour of a gas may be studied in terms of a quantity ‘z’called compressibility factor which is defined as z=PV/n. RT
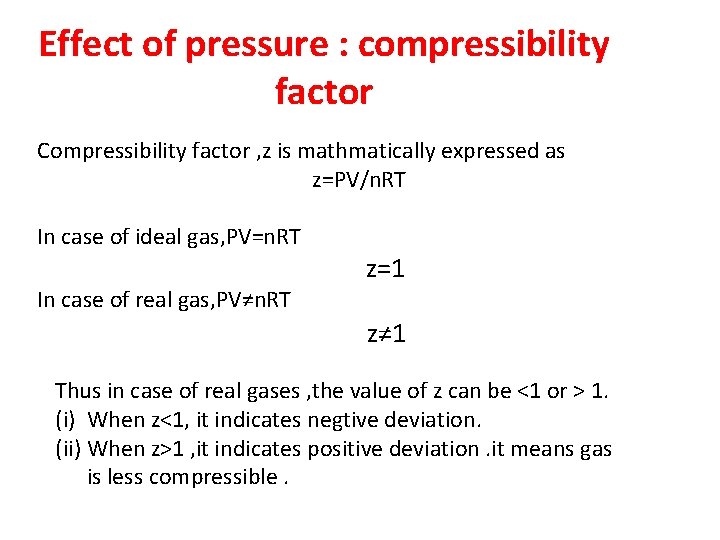
Effect of pressure : compressibility factor Compressibility factor , z is mathmatically expressed as z=PV/n. RT In case of ideal gas, PV=n. RT In case of real gas, PV≠n. RT z=1 z≠ 1 Thus in case of real gases , the value of z can be <1 or > 1. (i) When z<1, it indicates negtive deviation. (ii) When z>1 , it indicates positive deviation. it means gas is less compressible.
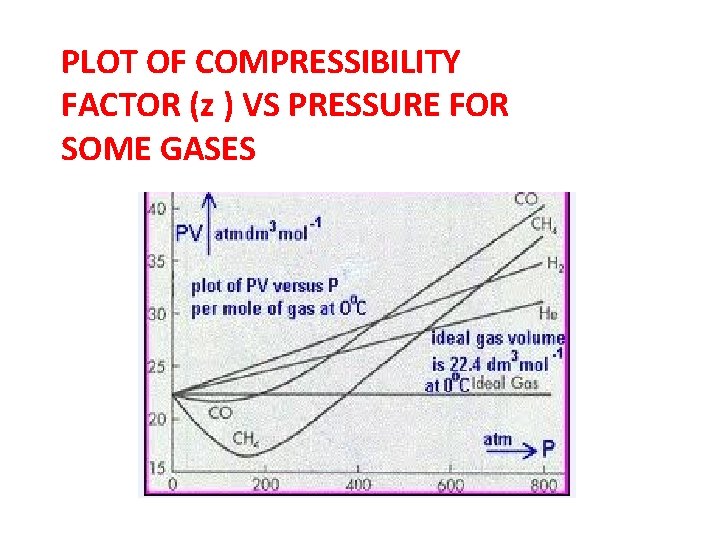
PLOT OF COMPRESSIBILITY FACTOR (z ) VS PRESSURE FOR SOME GASES
EFFECT OF TEMPERATURE: BOYLE TEMPERATURE The deviation from ideal behaviour become less and less with increase in temperature. The temperature at which a real gas behave like an ideal gas is called boyle’s temperature. The boyle temperature is different for different gases.
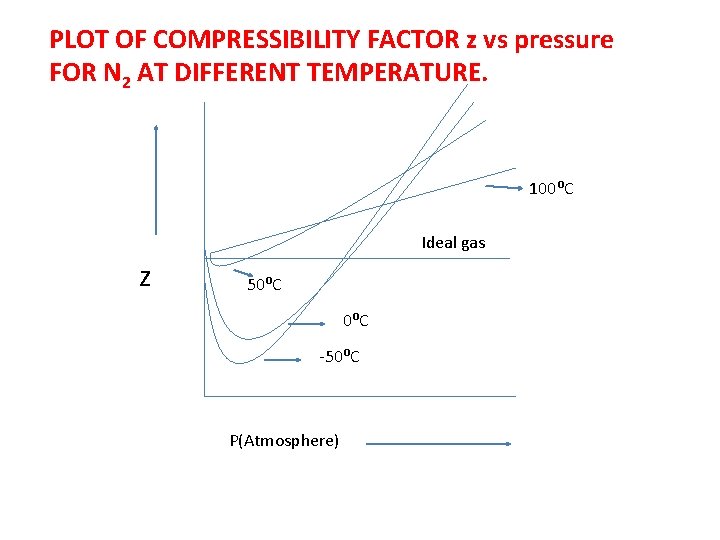
PLOT OF COMPRESSIBILITY FACTOR z vs pressure FOR N 2 AT DIFFERENT TEMPERATURE. 100⁰C Ideal gas z 50⁰C -50⁰C P(Atmosphere)
- Slides: 9