Colligative Properties Freezing Point Depression Boiling Point Elevation

Colligative Properties Freezing Point Depression Boiling Point Elevation Vapor Pressure Lowers

Colligative Properties • • Properties that depend ONLY on the number of solute particles Do NOT depend on their identity
Ionic vs. Molecular Solutes �Ionic solutes produce two or more ion particles in solution Na. Cl Na+ + Cl�Molecular solutes (covalent compounds) do not dissociate in solution C 2 H 6 �Ionic solutes have more particles, so they effect the colligative properties more than molecular solutes. �The effect is proportional to the number of particles of the solute in the solution.

Learning Check How many particles do each of the following give upon solvation? �Na. Cl �Ca. Cl 2 �Glucose (C 6 H 12 O 6)
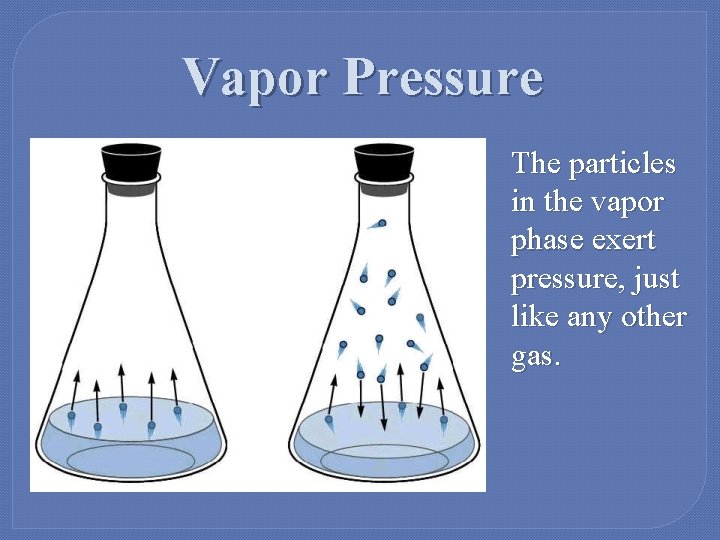
Vapor Pressure The particles in the vapor phase exert pressure, just like any other gas.

Vapor Pressure Lowering �The solute particles are surrounded by and attracted to particles of solvent. WHY? �This reduces the kinetic energy of the solvent particles, causing fewer particles to escape into the space above the liquid. �The result is a lower vapor pressure.

Freezing Point Depression When a solute is added to a pure liquid, the resulting solution has a lower freezing point than the pure liquid. Ex: Na. Cl (salt) added to water Water freezes at 0 o. C, but when salt is added it will not freeze until a negative temperature. The more salt that is added, the lower the temperature must be.

Boiling Point Elevation When a solute is added to a pure liquid, the resulting solution has a higher boiling point than the pure liquid. Ex: Na. Cl (salt) added to water Water boils at 100 o. C, but when salt is added it will not boil until a higher temperature. The more salt that is added, the higher the temperature must be.
- Slides: 8