Collection and Transport of Specimens for Microbiological Examinations

Collection and Transport of Specimens for Microbiological Examinations (Physician Assistants , Nurses & Public Health Students) INNOCENT AFEKE SCH. OF ALLIED HEALTH SCIENCES UHAS

Philosophy: The result of any laboratory examination is only as good as the sample received in the laboratory Think about this

Upon completion of this topic, the student should be able to: • Emphasize that obtaining sensitive and specific microbiology results begins with the patient and not at the door of the microbiology laboratory • Highlight the importance of proper collection and transport of specimens in both local and referral environments • Stress the importance of timely communication between the Microbiology laboratory and those collecting specimens • Describe common pitfalls in specimen collection and transport • Discuss the 7 rules or principles one must be followed in order to collect microbiology specimens which will accurately reflect the pathogenesis of the microbiological agent
Introduction • Specimens submitted for Microbi testing require proper handling from the time of collection through all stages of transport, storage and processing • Nurses and PAs are most often delegated to carry out these processes and hence needed to understand the requirements and consequences of such procedures • There may be prior preparation of patient before collection of specimen • Once the specimen is collected, it should be IMMEDIATELY dispatched to the Lab -delay in sending the specimen may cause delicate microbes to die from; Lack of nutrition, lowered or raised temperature, action of enzymes etc.
Influences of Sample Management Influences the accuracy of laboratory diagnosis Influences laboratory efficiency Good sample managemen t affects patient care and outcome Influences therapeutic decisions
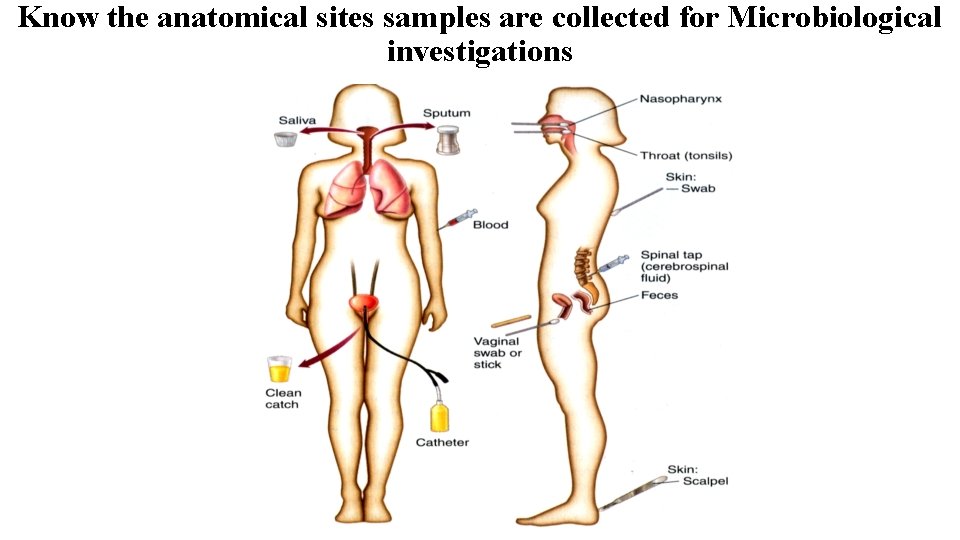
Know the anatomical sites samples are collected for Microbiological investigations

Samples that are Collected for Microbiological Testing BLOOD for: • Culture -preparation of site: critical -Multiple specimens: different sites -Bottle kept at RT or 35 -37°C (Do not refrigerate) • Serology -Serum/ Plasma: choice of bottle - Store frozen at -70°C

Field Work: Blood Collection & transport • Collection of blood from large communities for research -Dried Blood Spot for serology - Blood film on slide for parasitology - Unless necessary, take blood into sample tubes
CEREBROSPINAL FLUID (CSF) for: • Cytology -first tapped specimen • Biochemistry -Glucose & Protein -2 nd tapped specimen • Microbiology; culture -3 rd tapped specimen -about 3 ml NB CSF is emergency specimen and must be sent to lab immediately (No refrigeration except for viral cultures) • Specimen collection is done by specialist

URINE for: SPECIAL CASES: • Routine examination (RE) for microorganisms e. g. - • Paediatric urine collection kits are Trichomonas vaginalis, Schistosoma ova, Candida also available etc. -Collected into clean & dry universal bottle • Culture and Sensitivity Testing (CS) -Patient preparation very critical -Clean mid-stream catch urine • Suprapubic Aspiration -Sterile universal bottle 2/3 full Specimen: needle aspiration -Immediately refrigerate the specimen and submit through the abdominal wall into to the lab within 24 hours of collection (maintain at the bladder 2 -8 ºC when transporting) • Immunology(ELISA) & Molecular testing (PCR) -First early morning voided specimen • Catheter Collection Specimen can be used specially when patient cannot produce urine by him/her self
Field Work : Urine sample • For immunology test e. g. ELISA and for Molecular test e. g. PCR ; for organisms like N. gonorrhoea, Chlamydia and viruses that are shared in urine -Take sample into conning tube and store on ice whilst on the field -Transport to the lab and store at -20 o. C till ready to work on them • Urine samples for parasitology e. g. Schistosoma ova - Preserve with 10% formalin and store at RT • Conning Tubes
STOOL for: • FIELD WORK • RE for parasites e. g. amoeba, Tape worm, • Immunology & PCR Hookworm etc. -For Ag detection/PCR – no -clean wide-mouth crew-capped container transport medium -work on immediately or store @ RT for -Transport on dry ice for Ag, PCR months in fixative(buffered formalin) detection • C&S - Store at -15 o. C for Ag detection o. C if longer and PCR; , -70 -Freshly passed stool samples Øavoid specimens from a bed pan Rectal swabs unless sterile one Advantage: convenient, adapted to small children, debilitated patients -Use sterile or clean container and other situations where voided Ødo not clean with disinfectant stool sample not feasible -Cary-Blair medium (transport medium) Drawbacks: no macroscopic o -Storage &Transport: refrigerate at 4 C if assessment possible & less testing within 48 hours material available
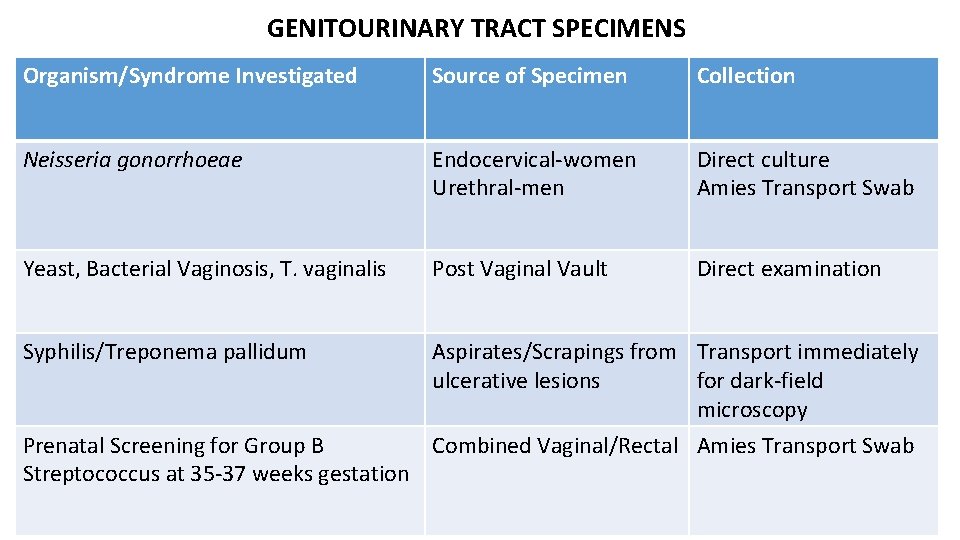
GENITOURINARY TRACT SPECIMENS Organism/Syndrome Investigated Source of Specimen Collection Neisseria gonorrhoeae Endocervical-women Urethral-men Direct culture Amies Transport Swab Yeast, Bacterial Vaginosis, T. vaginalis Post Vaginal Vault Direct examination Syphilis/Treponema pallidum Aspirates/Scrapings from Transport immediately ulcerative lesions for dark-field microscopy Prenatal Screening for Group B Combined Vaginal/Rectal Amies Transport Swab Streptococcus at 35 -37 weeks gestation
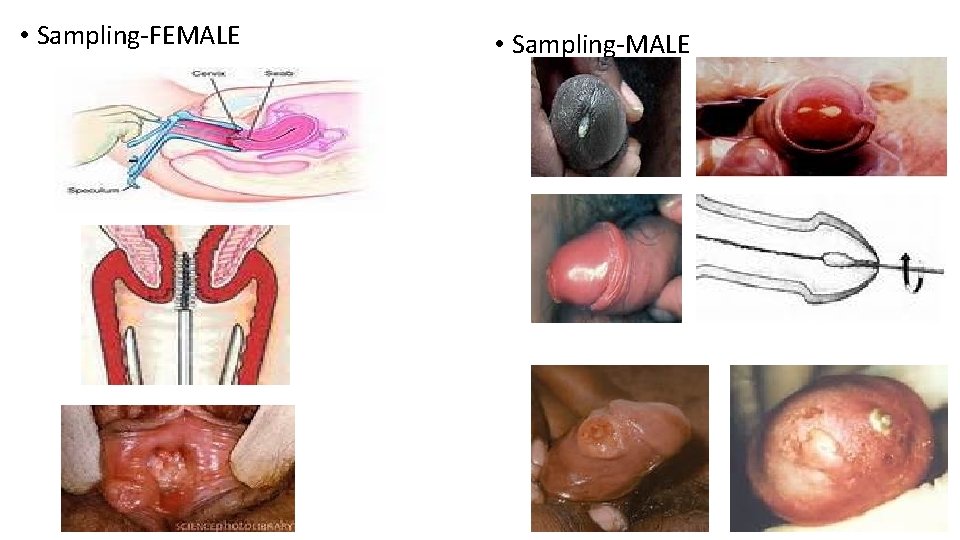
• Sampling-FEMALE • Sampling-MALE

RESPIRATORY SPECIMENS a) Lower Respiratory: Sputum for TB Culture • Do not rinse mouth with water as saprophytic mycobacteria in tap water may produce false positive results • Collect an early morning specimen from a deep, productive cough on three consecutive days into the empty 90 m. L sterile or clean container (? Ghana) • Carefully and tightly replace the cap • Wash your hands after collecting the specimen • Do not pool specimens • Store refrigerated at 2 -8°C • Submit to the PHL laboratory within 24 hours of collection with a completed PHL requisition
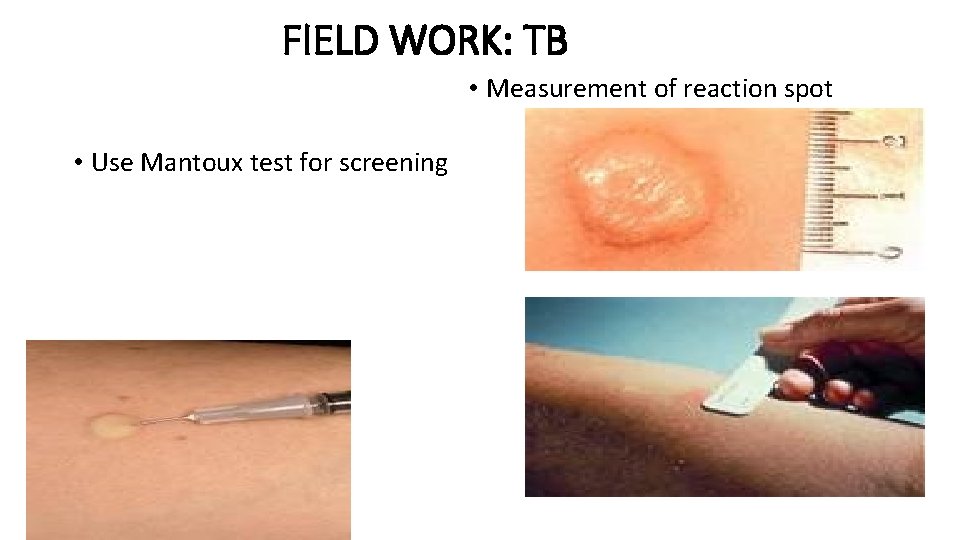
FIELD WORK: TB • Measurement of reaction spot • Use Mantoux test for screening

b) Upper Respiratory: Throat swab (posterior pharyngeal swab) -Hold tongue away with tongue depressor -Locate areas of inflammation and exudate in posterior pharynx, tonsillar region of throat behind uvula -Avoid swabbing soft palate; do not touch tongue -Rub area back and forth with cotton or Dacron swab WHO/CDS/EPR/ARO/2006. 1

c) Upper Respiratory: Nasopharyngeal swab -Tilt head backwards -Insert flexible fine-shafted polyester swab into nostril and back to nasopharynx -Leave in place a few seconds -Withdraw slowly; rotating motion -This procedure is done by a trained physician
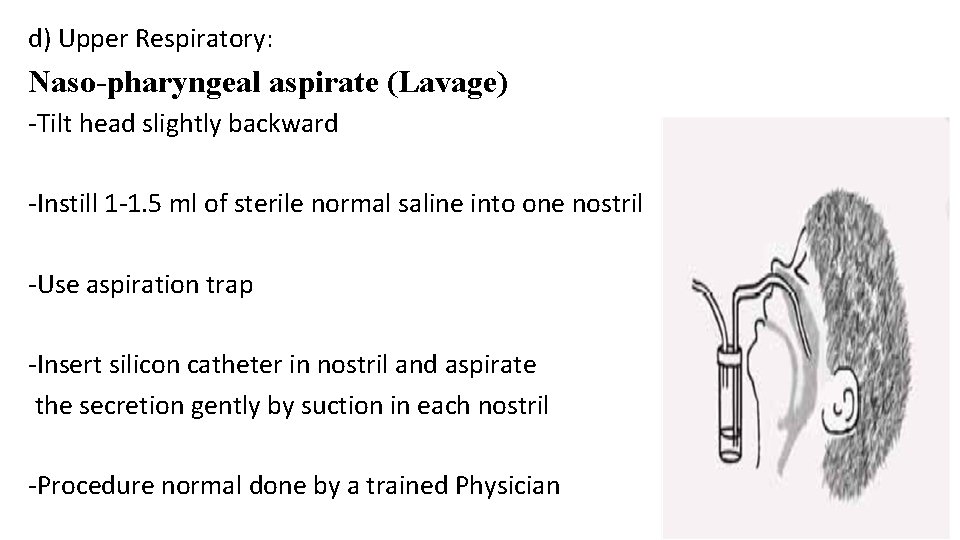
d) Upper Respiratory: Naso-pharyngeal aspirate (Lavage) -Tilt head slightly backward -Instill 1 -1. 5 ml of sterile normal saline into one nostril -Use aspiration trap -Insert silicon catheter in nostril and aspirate the secretion gently by suction in each nostril -Procedure normal done by a trained Physician
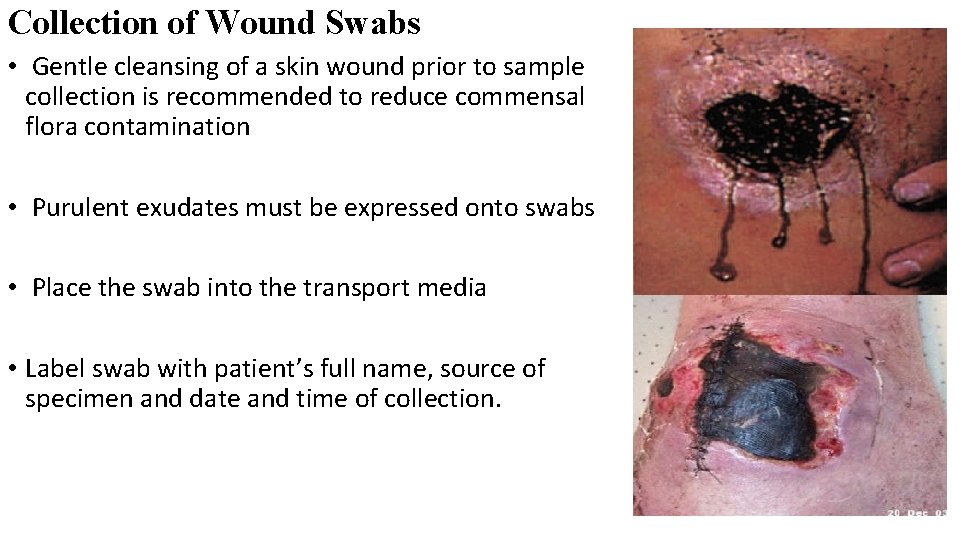
Collection of Wound Swabs • Gentle cleansing of a skin wound prior to sample collection is recommended to reduce commensal flora contamination • Purulent exudates must be expressed onto swabs • Place the swab into the transport media • Label swab with patient’s full name, source of specimen and date and time of collection.
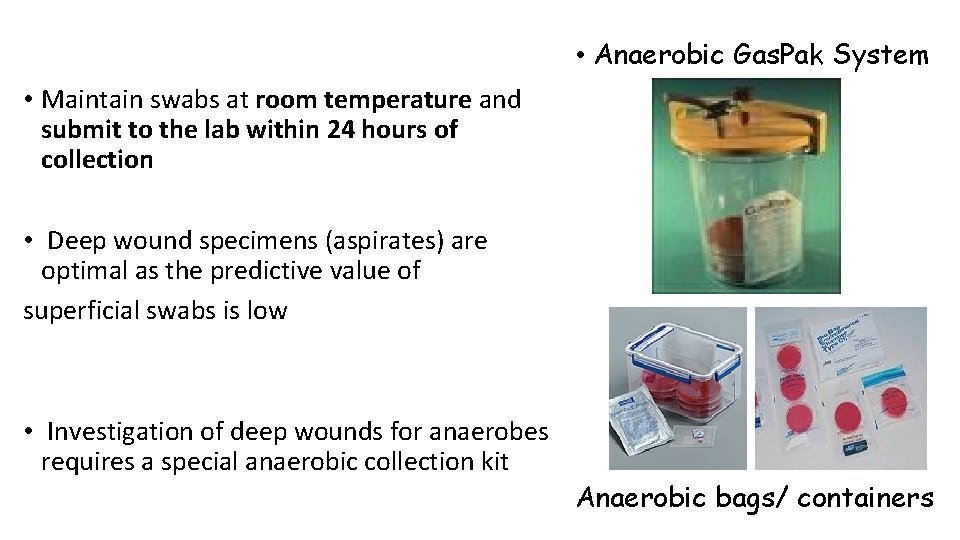
• Anaerobic Gas. Pak System • Maintain swabs at room temperature and submit to the lab within 24 hours of collection • Deep wound specimens (aspirates) are optimal as the predictive value of superficial swabs is low • Investigation of deep wounds for anaerobes requires a special anaerobic collection kit Anaerobic bags/ containers

Tissue for Culture • Tissue for routine C&S should be collected in a sterile container • For small samples add several drops of sterile saline to the container to maintain moisture • Maintain at room temperature and submit to the laboratory within 24 hours of collection
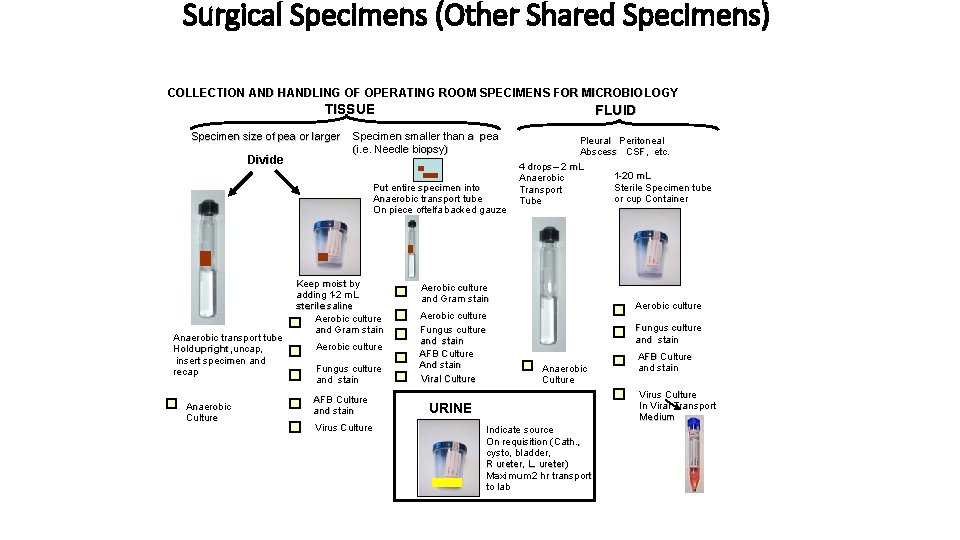
Surgical Specimens (Other Shared Specimens) COLLECTION AND HANDLING OF OPERATING ROOM SPECIMENS FOR MICROBIO LOGY TISSUE Specimen size of pea or larger Divide FLUID Specimen smaller than a pea (i. e. Needle biopsy) Put entire specimen into Anaerobic transport tube On piece oftelfa backed gauze Anaerobic transport tube Hold upright, uncap, insert specimen and recap Anaerobic Culture Keep moist by adding 11 -2 m. L sterile saline Aerobic culture and Gram stain Aerobic culture Fungus culture and stain AFB Culture and stain Virus Culture Pleural Peritoneal Abscess CSF, etc. 4 drops – 2 m. L 1 -20 m. L Anaerobic Sterile Specimen tube Transport or cup Container Tube Aerobic culture and Gram stain Aerobic culture Fungus culture and stain AFB Culture And stain Viral Culture Aerobic culture Fungus culture and stain Anaerobic Culture AFB Culture and stain Virus Culture In Viral Transport Medium URINE Indicate source On requisition (Cath. , cysto, bladder, R ureter, L. ureter) Maximum 2 hr transport to lab

Know the 7 rules or principles must be followed in order to collect microbiology specimens which will accurately reflect the pathogenesis of the microbiological agent
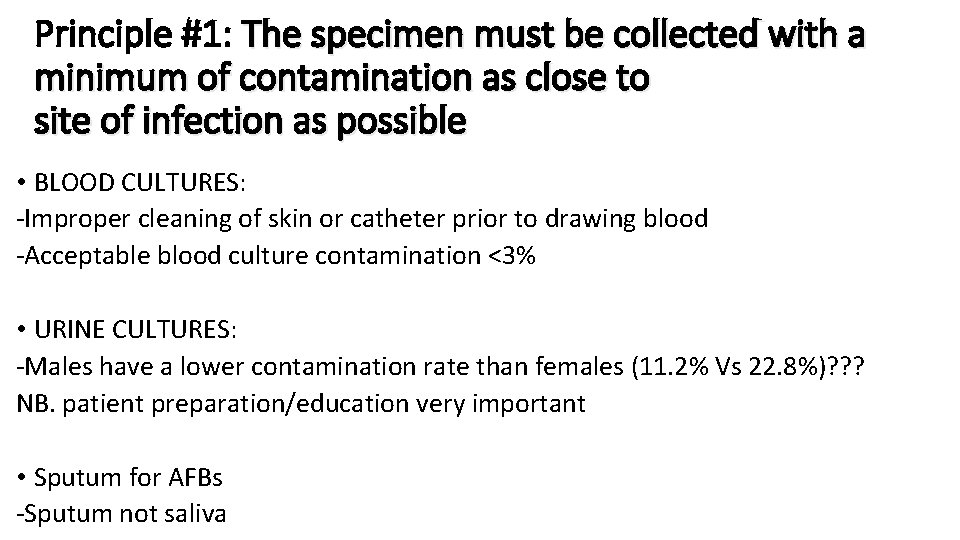
Principle #1: The specimen must be collected with a minimum of contamination as close to site of infection as possible • BLOOD CULTURES: -Improper cleaning of skin or catheter prior to drawing blood -Acceptable blood culture contamination <3% • URINE CULTURES: -Males have a lower contamination rate than females (11. 2% Vs 22. 8%)? ? ? NB. patient preparation/education very important • Sputum for AFBs -Sputum not saliva
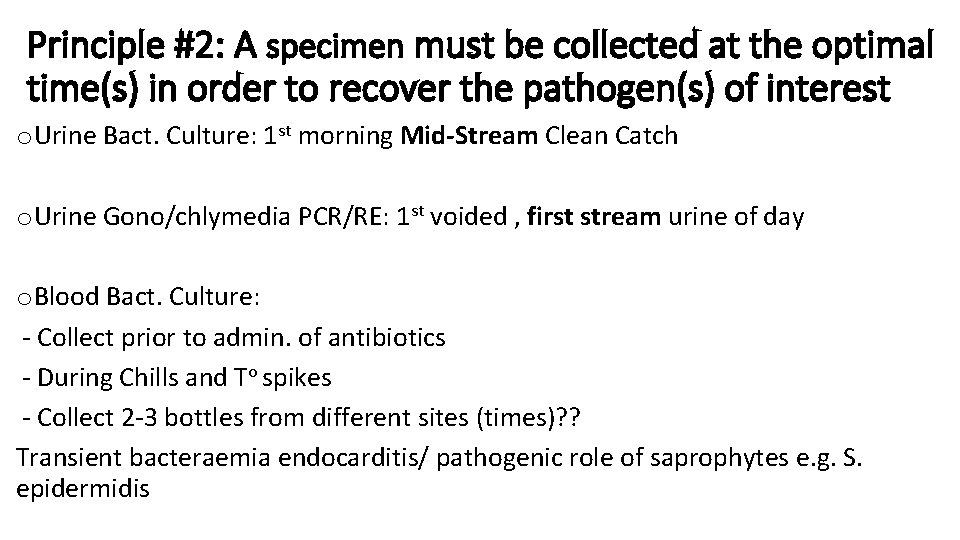
Principle #2: A specimen must be collected at the optimal time(s) in order to recover the pathogen(s) of interest o Urine Bact. Culture: 1 st morning Mid-Stream Clean Catch o Urine Gono/chlymedia PCR/RE: 1 st voided , first stream urine of day o Blood Bact. Culture: - Collect prior to admin. of antibiotics - During Chills and To spikes - Collect 2 -3 bottles from different sites (times)? ? Transient bacteraemia endocarditis/ pathogenic role of saprophytes e. g. S. epidermidis

o Blood Parasites: Collect during febrile episode or every 6 hrs for 24 hrs o Sputum for AFBs Microscopy & Culture: -3 consecutive specimen collected 8 -24 hrs apart -At least one must be early morning specimen -In Ghana, one early morning + One spot specimen at clinic/Lab. o Viral cultures: Collect as soon after onset of symptoms as possible o Stool specimen a) For parasites ; vegetative form e. g. Entamoeba histolytica: freshly stool specimen b) For ova, cysts and Oocyst: preserve stool within an hour of collection

Principle #3: A sufficient quantity of the specimen must be obtained to perform the requested tests • BLOOD -Adult : 10 ml for aerobic, 10 ml for anaerobic bottle -Child: 2 -5 ml -Infant: 0. 5 -2 ml why? ? • CSF -At least 0. 5 ml in 1 st bottle, 2 ml each into bottles 2 &3 • URINE - At least 12 ml (2/3 of the container, do not fill it)

Principle #4: Appropriate collection devices and specimen containers must be used to ensure recovery of specific organisms • Anaerobic Culture -Best collected with metal; needle aspiration or with a scalpel • Viral culture sample must not be transported in bacterial transport media • Skin Parasites: place skin scraping in a clean dry container, cap tightly and transport to lab within 24 hrs at RT • Blood culture from Heparin or EDTA tube -Heparin is toxic to many organisms -Transferring from these tubes increases risk of contamination

Principle #5: Collect all microbiology test samples prior to the institution of antimicrobial agents • Hair, Skin and Nails for fungal culture: -Collect sample before antifungal therapy -if already started, discontinue at least 5 days before taken sample • Urine Culture: -Antibiotics may cause a transient decrease in bacterial concentration resulting in a false negative report • Blood Culture: - Take blood sample before antibiotic therapy - State on the lab form if you have started therapy before blood taken
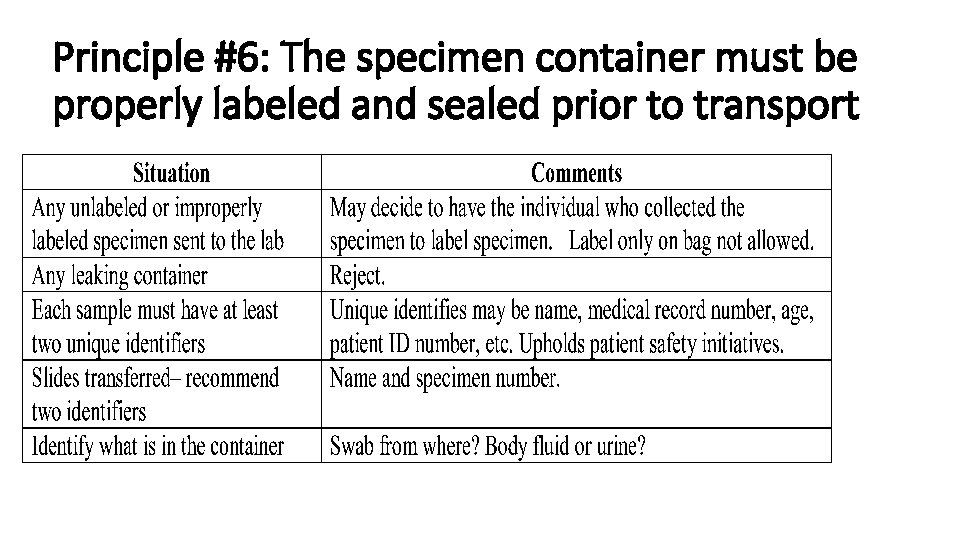
Principle #6: The specimen container must be properly labeled and sealed prior to transport
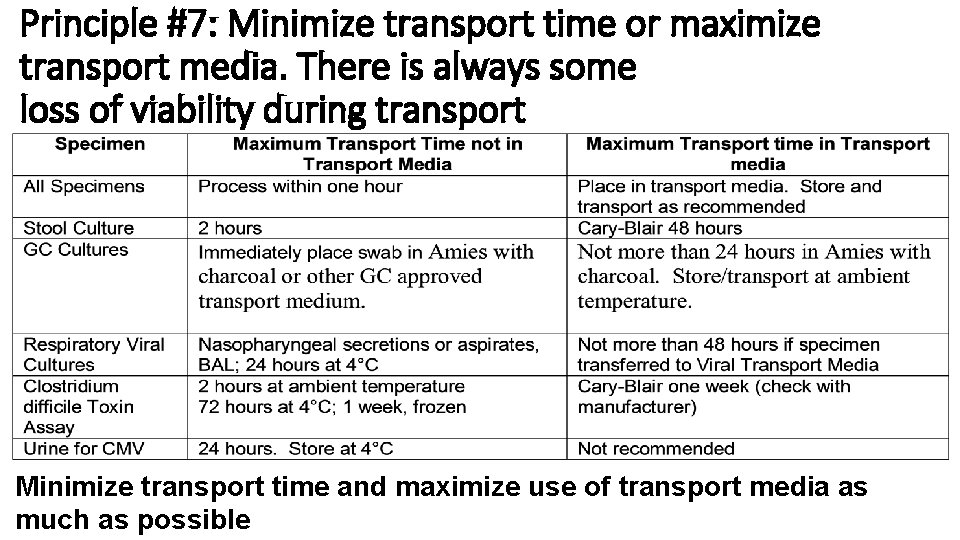
Principle #7: Minimize transport time or maximize transport media. There is always some loss of viability during transport Minimize transport time and maximize use of transport media as much as possible
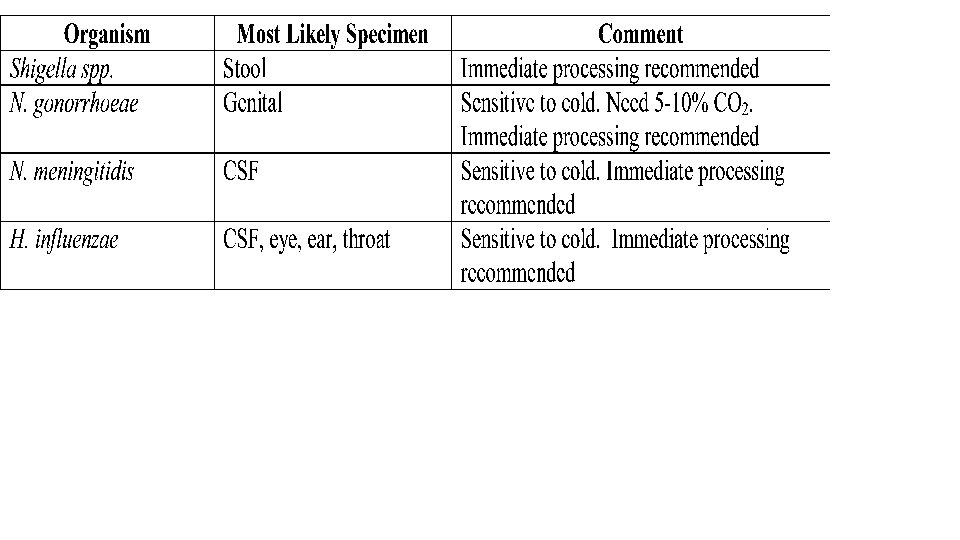
Criteria For Rejection of Microbiological Specimens • Unlabeled or improperly labeled specimen • Prolonged storage or transport • Improper or damaged container • Specimen received in fixative • Oropharyngeal contaminated sputum • Duplicate specimens stools, sputum) within a 24 hour period. Exceptions cleared by the laboratory • Specimens unsuitable for culture request (anaerobic culture from not acceptable source, urine from Foley catheter) • Dry Swab • 24 -hr collection of urine or sputum for AFB or fungal culture
- Slides: 34