Cold Working is Actually Strain Hardening Basic equation
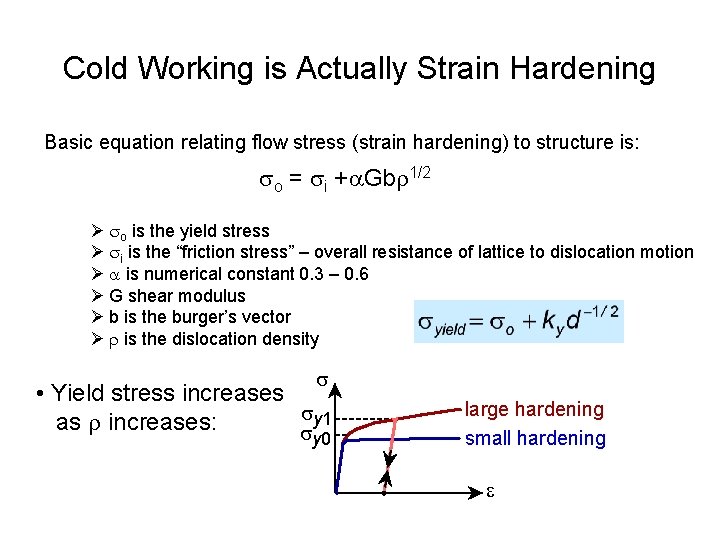
Cold Working is Actually Strain Hardening Basic equation relating flow stress (strain hardening) to structure is: so = si +a. Gbr 1/2 Ø so is the yield stress Ø si is the “friction stress” – overall resistance of lattice to dislocation motion Ø a is numerical constant 0. 3 – 0. 6 Ø G shear modulus Ø b is the burger’s vector Ø r is the dislocation density s • Yield stress increases sy 1 as r increases: s y 0 large hardening small hardening e
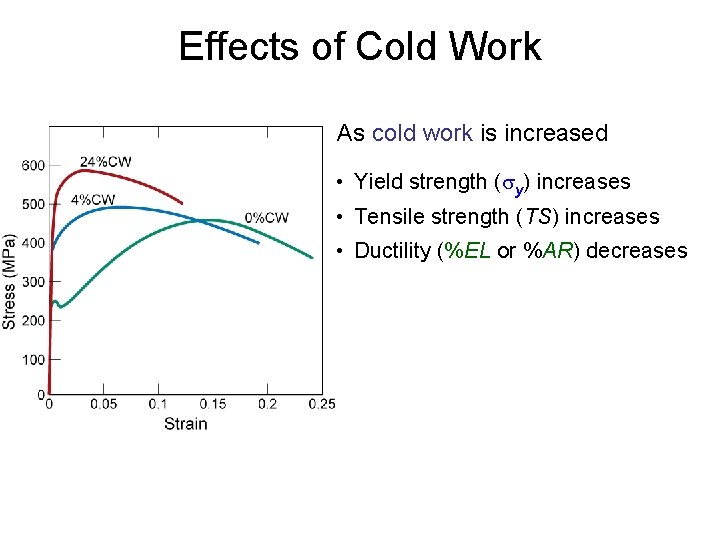
Effects of Cold Work As cold work is increased • Yield strength (sy) increases • Tensile strength (TS) increases • Ductility (%EL or %AR) decreases

Other Cold Work Effects • Usually a small decrease in density (few 10 ths of a percent) • An appreciable decrease in electrical conductivity (increased number of scattering centers) • Small increase in thermal coefficient of expansion • Because of increased internal energy – chemical reactivity is increased (decreased resistance to corrosion)
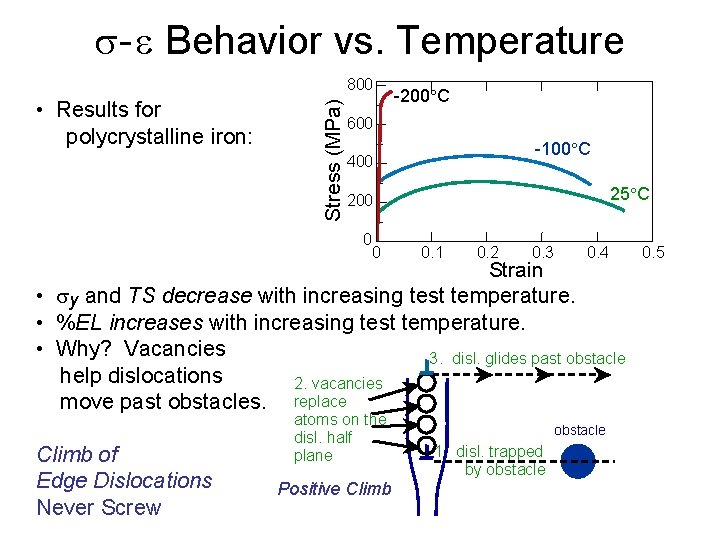
s- e Behavior vs. Temperature • Results for polycrystalline iron: Stress (MPa) 800 -200 C 600 -100 C 400 25 C 200 0 0 0. 1 0. 2 0. 3 0. 4 Strain • sy and TS decrease with increasing test temperature. • %EL increases with increasing test temperature. • Why? Vacancies 3. disl. glides past obstacle help dislocations 2. vacancies move past obstacles. replace Climb of Edge Dislocations Never Screw atoms on the disl. half plane Positive Climb obstacle 1. disl. trapped by obstacle 0. 5

Strain Energy Related to Cold Work Figure: Stored energy of cold work and fraction of the total work of deformation remaining as stored energy for high purity copper • Mentioned that ~10% of the energy imparted during cold working is stored as strain energy • Amount of strain energy is increased by increasing the severity of deformation, lowering the deformation temperature, and by impurity additions • The strain energy increase is stored in the highly deformed microstructure – dislocation tangles • Metastable microstructure! Source: Reed-Hill & Abbaschian, Physical Metallurgy Principles, 3 rd Edition, PWS Publishing Company, 1994.

Annealing • Can we release the stored strain energy? YES! • The material is in an unstable state – but there is an activation energy barrier to releasing that energy • By heating the material and adding energy to the system we can increase the probability of moving past the activation barrier • Heat treating cold worked material is called Annealing
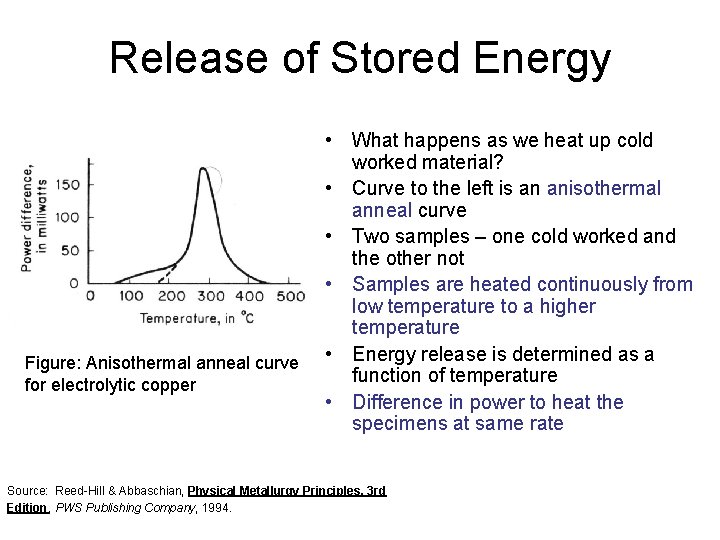
Release of Stored Energy Figure: Anisothermal anneal curve for electrolytic copper • What happens as we heat up cold worked material? • Curve to the left is an anisothermal anneal curve • Two samples – one cold worked and the other not • Samples are heated continuously from low temperature to a higher temperature • Energy release is determined as a function of temperature • Difference in power to heat the specimens at same rate Source: Reed-Hill & Abbaschian, Physical Metallurgy Principles, 3 rd Edition, PWS Publishing Company, 1994.
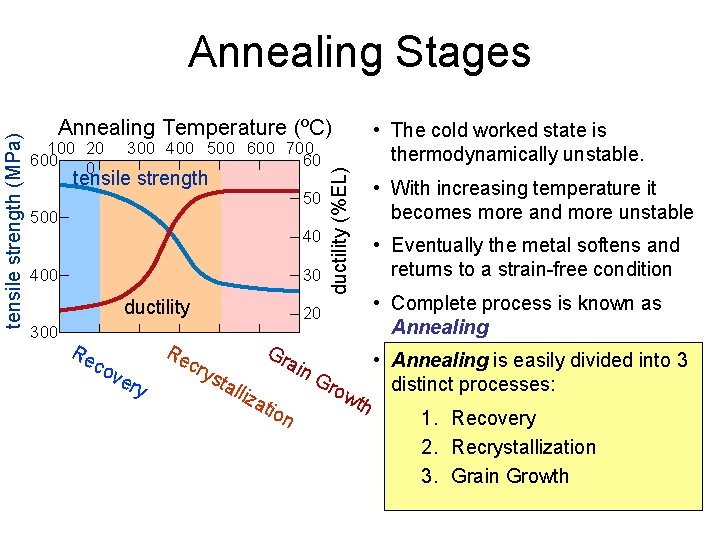
Annealing Temperature (ºC) 100 20 600 0 300 400 500 600 700 60 tensile strength 50 500 40 400 30 ductility 300 Re co ve ry Re c 20 Gr ai ductility (%EL) tensile strength (MPa) Annealing Stages • The cold worked state is thermodynamically unstable. • With increasing temperature it becomes more and more unstable • Eventually the metal softens and returns to a strain-free condition • Complete process is known as Annealing • Annealing is easily divided into 3 n. G tal distinct processes: row liza th tio n 1. Recovery 2. Recrystallization 3. Grain Growth rys
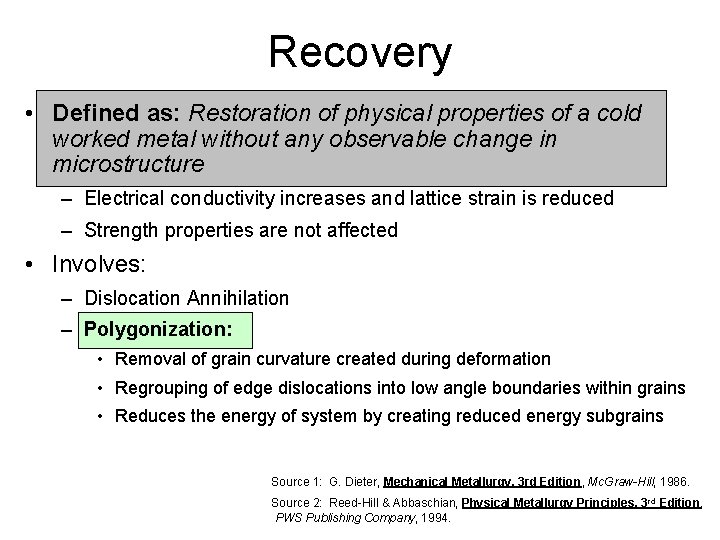
Recovery • Defined as: Restoration of physical properties of a cold worked metal without any observable change in microstructure – Electrical conductivity increases and lattice strain is reduced – Strength properties are not affected • Involves: – Dislocation Annihilation – Polygonization: • Removal of grain curvature created during deformation • Regrouping of edge dislocations into low angle boundaries within grains • Reduces the energy of system by creating reduced energy subgrains Source 1: G. Dieter, Mechanical Metallurgy, 3 rd Edition, Mc. Graw-Hill, 1986. Source 2: Reed-Hill & Abbaschian, Physical Metallurgy Principles, 3 rd Edition, PWS Publishing Company, 1994.
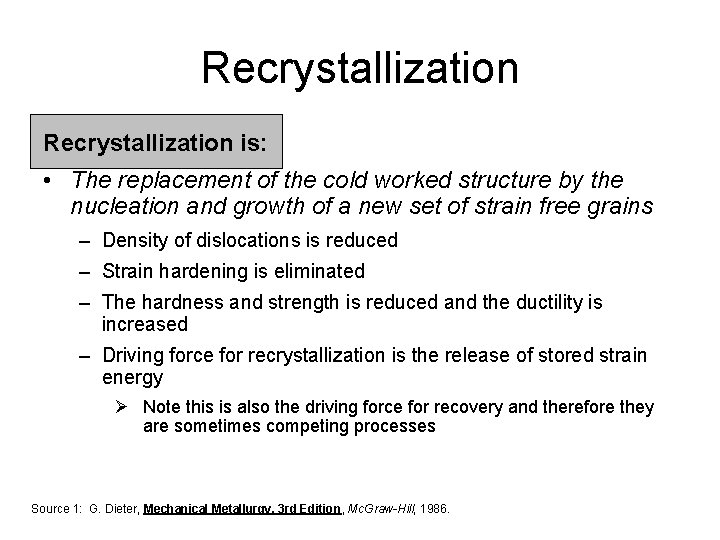
Recrystallization is: • The replacement of the cold worked structure by the nucleation and growth of a new set of strain free grains – Density of dislocations is reduced – Strain hardening is eliminated – The hardness and strength is reduced and the ductility is increased – Driving force for recrystallization is the release of stored strain energy Ø Note this is also the driving force for recovery and therefore they are sometimes competing processes Source 1: G. Dieter, Mechanical Metallurgy, 3 rd Edition, Mc. Graw-Hill, 1986.
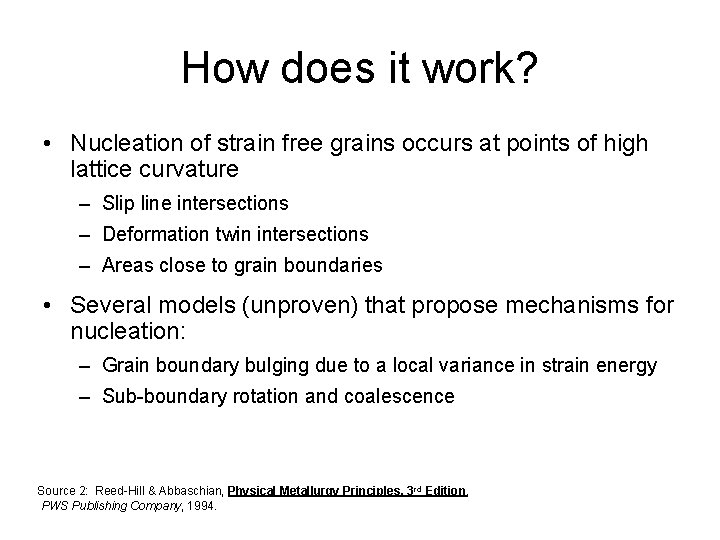
How does it work? • Nucleation of strain free grains occurs at points of high lattice curvature – Slip line intersections – Deformation twin intersections – Areas close to grain boundaries • Several models (unproven) that propose mechanisms for nucleation: – Grain boundary bulging due to a local variance in strain energy – Sub-boundary rotation and coalescence Source 2: Reed-Hill & Abbaschian, Physical Metallurgy Principles, 3 rd Edition, PWS Publishing Company, 1994.
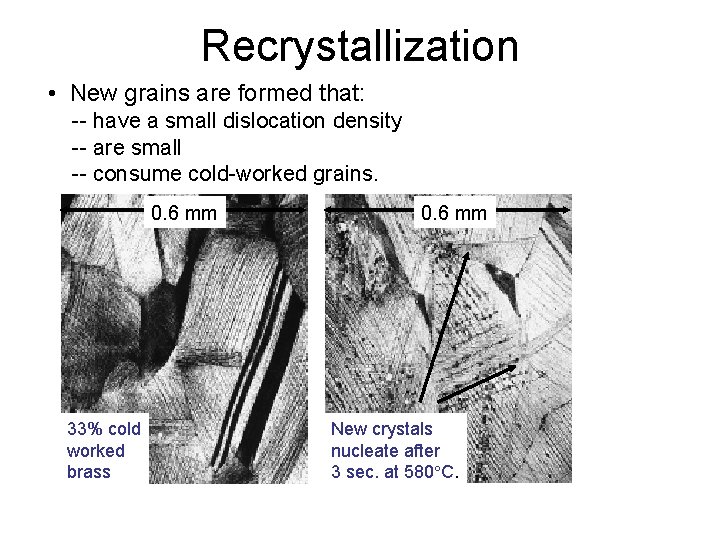
Recrystallization • New grains are formed that: -- have a small dislocation density -- are small -- consume cold-worked grains. 0. 6 mm 33% cold worked brass 0. 6 mm New crystals nucleate after 3 sec. at 580 C.
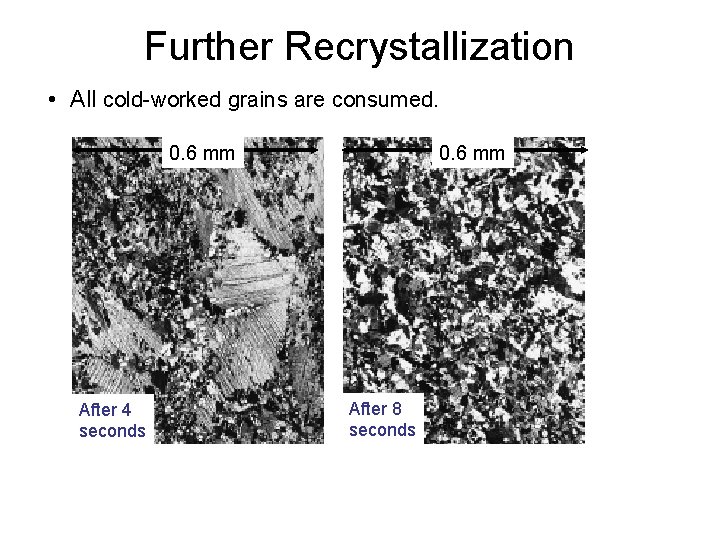
Further Recrystallization • All cold-worked grains are consumed. 0. 6 mm After 4 seconds 0. 6 mm After 8 seconds
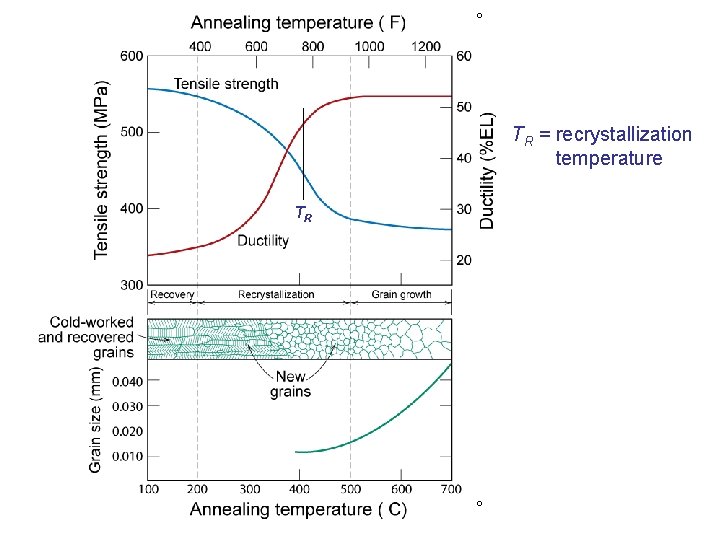
º TR = recrystallization temperature TR º
Variables for Recrystallization Six main variables influence recrystallization behavior: 1. 2. 3. 4. 5. 6. The amount of prior deformation Temperature Time Initial grain size Composition Amount of recovery or polygonization prior to the start of recrystallization Because the temperature at which recrystallization occurs depends Recrystallization temperature is not a fixed temperature like melting point The practical definition for recrystallization temperature is: The temperature at which a given alloy in a highly cold worked state completely recrystallizes in 1 hour. Source: G. Dieter, Mechanical Metallurgy, 3 rd Edition, Mc. Graw-Hill, 1986.
Affect of Variables on Recrystallization 1. Minimum amount of deformation is required 2. The smaller the deformation, the higher the temperature required for recrystallization 3. Increasing annealing time decreases required recrystallization temperature. Temperature is more important than time. Doubling annealing time is approximately equivalent to increasing annealing temperature 10 o. C 4. Final grain size depends most on the degree of deformation and to lesser extent on the annealing temperature. The greater the deformation & the lower the annealing temp. , the smaller the recrystallized grain size. 5. The larger the original grain size, the greater the amount of cold work required to produce same recrystallization temp. Source: G. Dieter, Mechanical Metallurgy, 3 rd Edition, Mc. Graw-Hill, 1986.
Affect of Variables on Recrystallization 6. 7. 8. The recrystallization temperature decreases with increasing purity of the metal. Solid solution alloying additions ALWAYS raise the recrystallization temperature. The amount of deformation required to produce equivalent recrystallization behavior increases with increased working temperature For a given reduction in cross-section – different metal working processes produce different effective deformations. Therefore, identical recrystallization behavior may not be obtained. Source: G. Dieter, Mechanical Metallurgy, 3 rd Edition, Mc. Graw-Hill, 1986.
- Slides: 17