Coil of wire compass battery The compass will


Coil of wire + compass battery + _ _ The compass will align with the coil of wire if current is flowing. Even though a battery is connected, no electrical current is flowing through the water.

Coil of wire + battery + compass _ _ Copper Chloride Copper chloride is added to the water. It dissolves in the water.
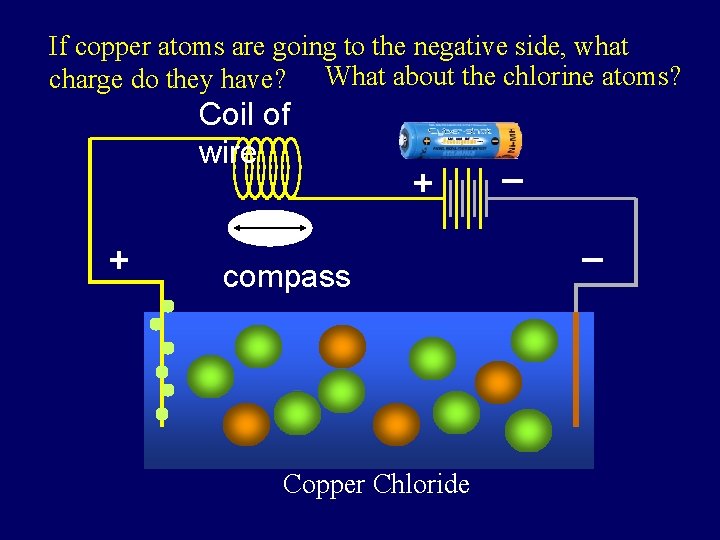
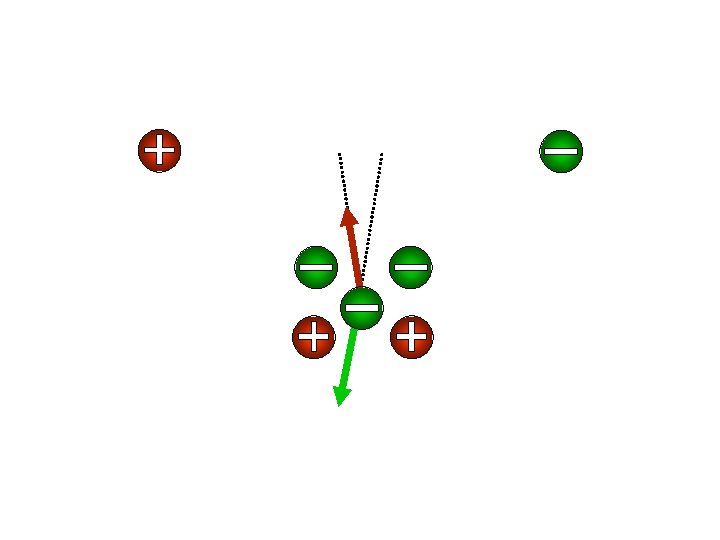
If copper atoms are going to the negative side, what charge do they have? What about the chlorine atoms? Coil of wire + battery + compass Copper Chloride _ _
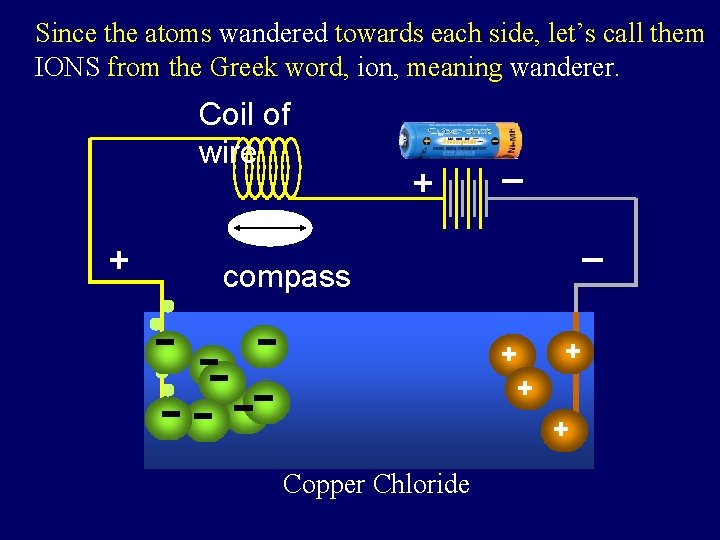
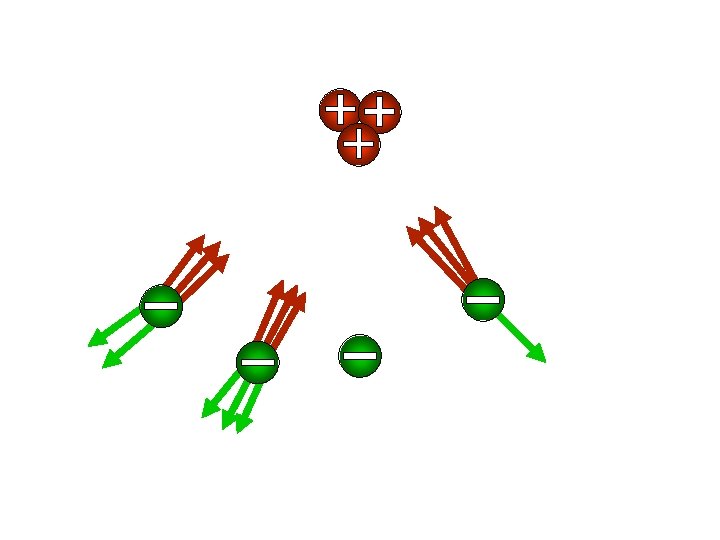
Since the atoms wandered towards each side, let’s call them IONS from the Greek word, ion, meaning wanderer. Coil of wire + battery + _ _ compass + + Copper Chloride
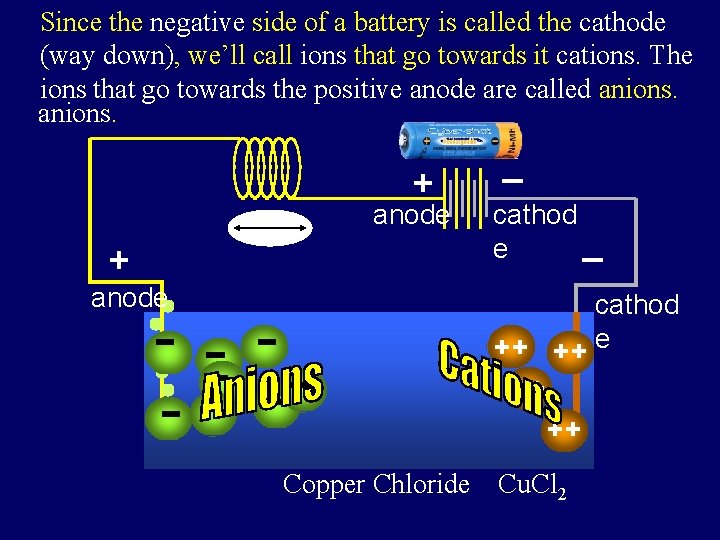
Since the negative side of a battery is called the cathode Also, since the positive side that of a go battery is called the The (way down), we’ll call ions towards it cations. anode (way we’llthe call ions that go towards it anions that go up), towards positive anode are called anions. + anode _ cathod _ e cathod ++ + + e ++ ++ Copper Chloride Cu. Cl 2
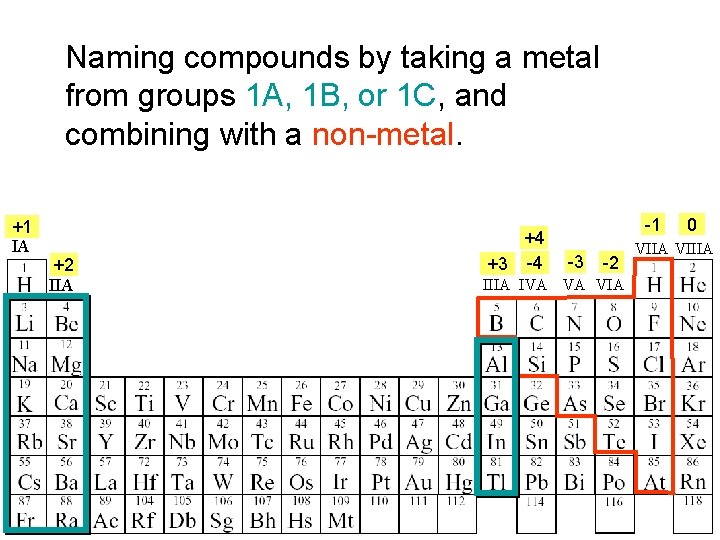
Naming compounds by taking a metal from groups 1 A, 1 B, or 1 C, and combining with a non-metal. +1 IA +2 IIA +4 +3 -4 -1 -3 -2 IIIA IVA VA VIA 0 VIIA VIIIA
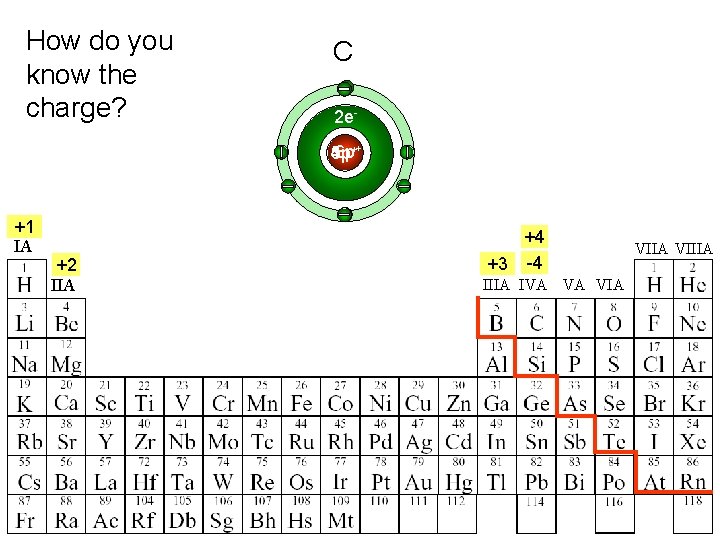
How do you know the charge? Be Li B C 2 e 6 p++ 5 p 3 p 4 p +1 IA +2 IIA +4 +3 -4 IIIA IVA VA VIIA VIIIA
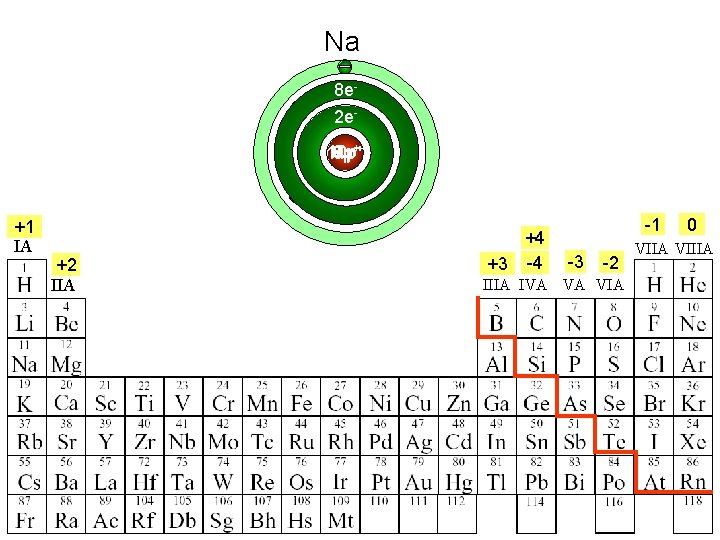
Ne Na O N F 8 e 2 e 8 p 10 p 11 p 7 p++ 9 p +1 IA +2 IIA +4 +3 -4 -1 -3 -2 IIIA IVA VA VIA 0 VIIA VIIIA
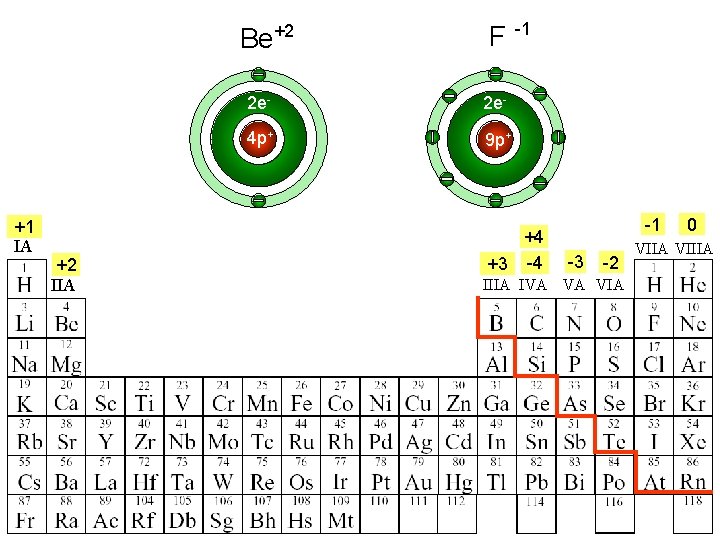
Be+2 +1 IA +2 IIA F -1 2 e- 4 p+ 9 p+ +4 +3 -4 -1 -3 -2 IIIA IVA VA VIA 0 VIIA VIIIA
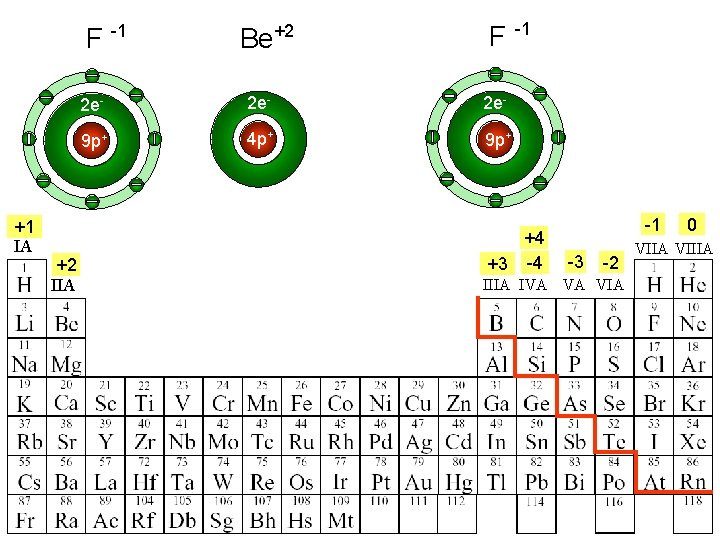
F -1 +1 IA +2 IIA Be+2 F -1 2 e- 2 e- 9 p+ 4 p+ 9 p+ +4 +3 -4 -1 -3 -2 IIIA IVA VA VIA 0 VIIA VIIIA
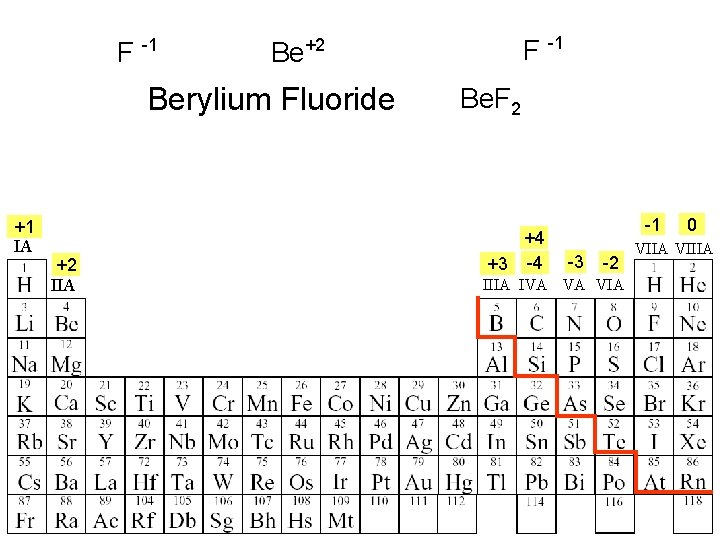
F -1 Berylium Fluoride +1 IA +2 IIA F -1 Be+2 Be. F 2 +4 +3 -4 -1 -3 -2 IIIA IVA VA VIA 0 VIIA VIIIA
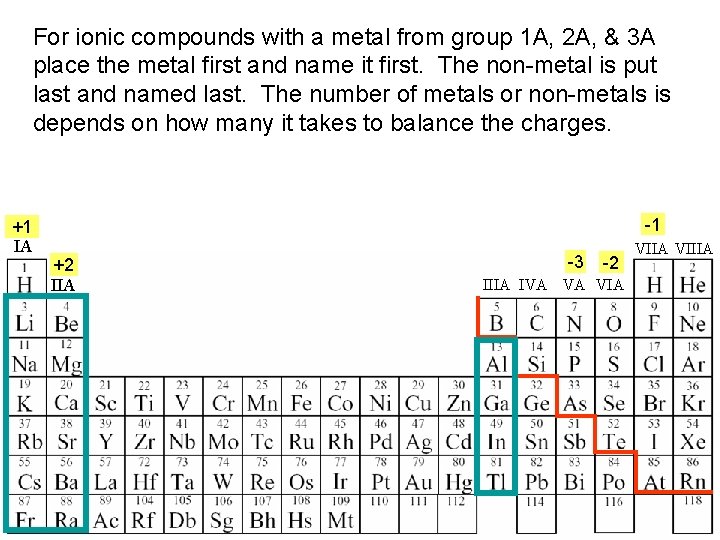
For ionic compounds with a metal from group 1 A, 2 A, & 3 A place the metal first and name it first. The non-metal is put last and named last. The number of metals or non-metals is depends on how many it takes to balance the charges. +1 IA -1 +2 IIA -3 -2 IIIA IVA VA VIIA VIIIA
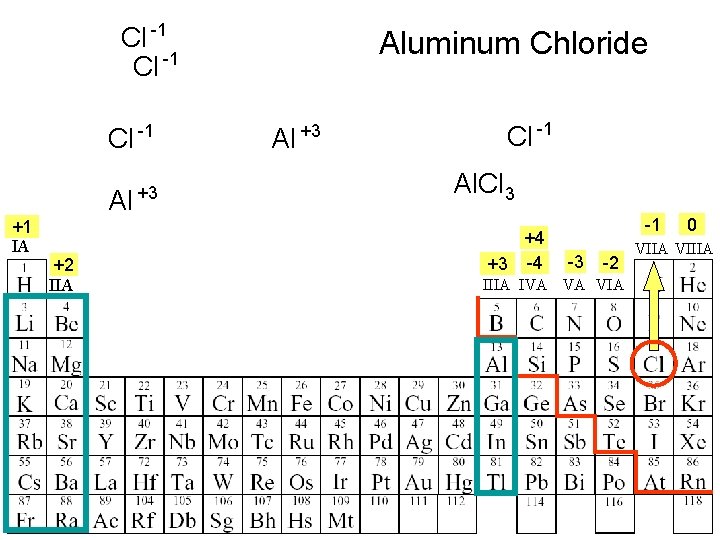
Cl -1 Al +1 IA +2 IIA +3 Aluminum Chloride Al +3 Cl -1 Al. Cl 3 +4 +3 -4 -1 -3 -2 IIIA IVA VA VIA 0 VIIA VIIIA
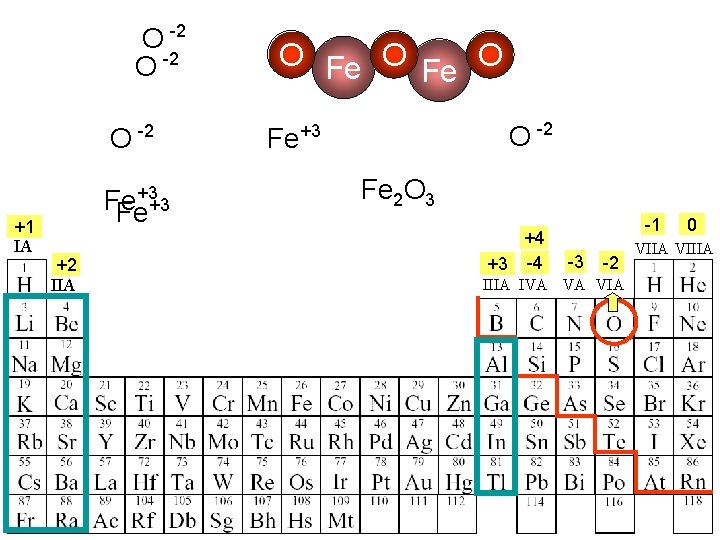
O -2 +1 IA +3 Fe Fe+3 +2 IIA O Fe O O -2 Fe+3 Fe 2 O 3 +4 +3 -4 -1 -3 -2 IIIA IVA VA VIA 0 VIIA VIIIA

The next slides show polyatomic ions and how they will balance their charges when combining with a metal.
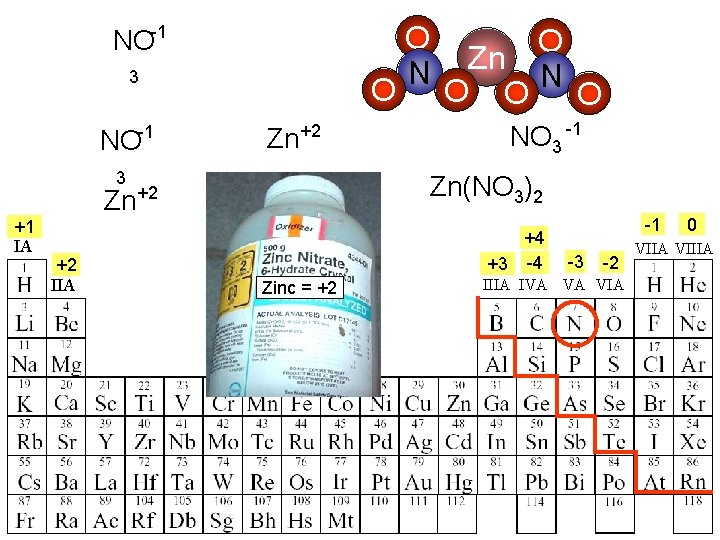
NO-1 3 -1 NO O Zn+2 3 +2 IIA Zn O O O NO 3 -1 Zn(NO 3)2 Zn+2 +1 IA O N +4 +3 -4 Zinc = +2 -1 -3 -2 IIIA IVA VA VIA 0 VIIA VIIIA
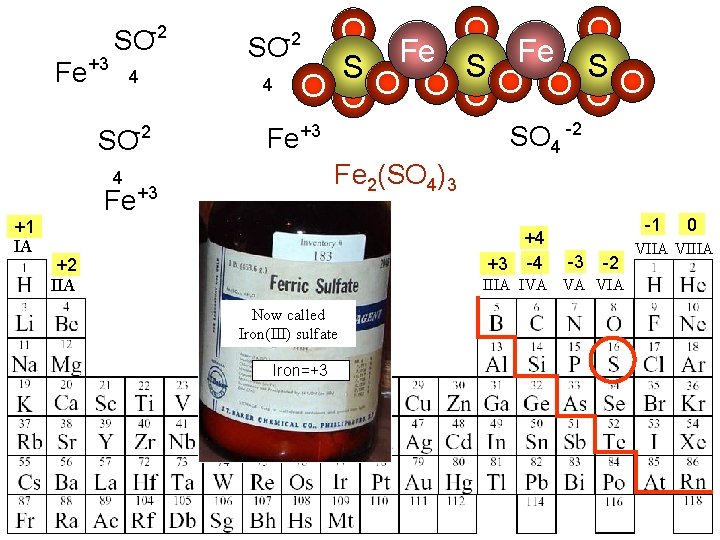
Fe+3 SO-2 4 -2 SO O SO Fe S S 4 O O O O O -2 Fe 2(SO 4)3 4 Fe+3 +1 IA SO 4 -2 Fe+3 +4 +3 -4 +2 IIA -1 -3 -2 IIIA IVA VA VIA Now called Iron(III) sulfate Iron=+3 0 VIIA VIIIA
- Slides: 20