Cohort Study Designs Ahmed Mandil Dept of Family





















- Slides: 28

Cohort Study Designs Ahmed Mandil Dept of Family & Community Medicine College of Medicine King Saud University

Headlines n n n Definitions Observational studies Cohort studies Advantages, disadvantages Examples Analysis 10/7/2020 Cohort Studies 2

Observation Methods n n Selected Units: individuals, groups Study Populations: cross-sectional, longitudinal Data collection timing: prospectively, retrospectively, combination Data collection types: primary, secondary 10/7/2020 Cohort Studies 3

Study populations n n Cross-sectional: where only ONE set of observations is collected for every unit in the study, at a certain point in time, disregarding the length of time of the study as a whole Longitudinal: where TWO or MORE sets of observations are collected for every unit in the study, i. e. follow-up is involved in order to allow monitoring of a certain population (cohort) over a specified period of time. Such populations are AT RISK (disease-free) at the start of the study. 10/7/2020 Cohort Studies 4

Study Designs in Health Research n Qualitative n n n Focus group discussions Key informant studies Ethnographic studies Bibliographic studies Others 10/7/2020 n Quantitative n Observational n Descriptive, e. g. cross -sectional n Analytical, e. g. casecontrol; cohort n Experimental (also analytical) n Randomized Clinical Trials n Community Intervention Studies Cohort Studies 5

Cohort Studies (I) (a) Characteristics: n A “cohort” is a group of people, referred to as “diseasefree population” or “population at risk” n A survey is first carried out to exclude prevalent cases from the cohort n A period of "follow-up“ is specified, for possible new cases' occurrence n We know the exposure status, looking for the disease status n Historical designs are preferred under occupational settings, for less frequent effects / exposures. 10/7/2020 Cohort Studies 6

Cohort Studies (II) Two types are recognized: n Prospective (longitudinal): forward in time follow-up study n Retrospective (historical): backward in time study (depends on records: medical / employment). This is the type preferred under occupational settings 10/7/2020 Cohort Studies 7

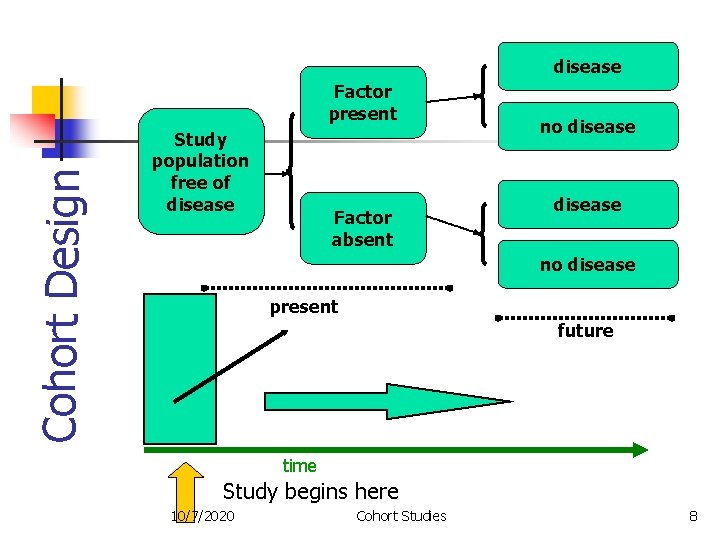
disease Cohort Design Factor present Study population free of disease Factor absent no disease present future time Study begins here 10/7/2020 Cohort Studies 8

Cohort Studies (III) (b) Advantages: n n n No / little temporal ambiguity (suggests cause-effect relationship) Calculation of incidence rates Suitable for rare exposures Factors associated with selection cannot influence disease status and hence the results. Several outcomes can be studied, after follow-up starts. 10/7/2020 Cohort Studies 9

Cohort Studies (IV) ( c ) Disadvantages (of prospective): n Expensive n Time-consuming n May be impractical n Loss to follow-up may affect samplesize 10/7/2020 Cohort Studies 10

IDEAL COHORT An ideal cohort should be: n STABLE n COOPERATIVE n COMMITTED n WELL-INFORMED 10/7/2020 Cohort Studies 11

Examples of Study Cohorts 1. 2. General population Selected occupational groups, e. g. health professionals (physicians, nurses, lab technologists, etc), manufacturers of mercury batteries for vehicles; asbestos workers, miners, etc 10/7/2020 Cohort Studies 12

Sources of Cohorts n n Population groups Occupational settings (employment, medical records) Hospital registers (medical records) Death certificates 10/7/2020 Cohort Studies 13

Follow-up Techniques 1. Periodical medical examinations and mailed questionnaires 2. Direct personal interviews or examinations 3. Videoconference, neighbors, friends and relatives 4. Lost persons can be traced through the letters, from their relatives and friends 5. Migrated cohort subjects can also be traced through travel and immigration authorities 6. Dead persons - local or regional mortality registers or death certificates 10/7/2020 Cohort Studies 14

Problems during Follow-up n n n FOLLOW-UP OF A LARGE GROUP LIMITED RESOURCES TIME SCARCITY PAUCITY OF TRAINED PERSONNEL ATTRITION, LOSS ON FOLLOW UP ETHICAL CONCERNS 10/7/2020 Cohort Studies 15

Attrition Reduction n n OBTAINING THE INFORMED CONSENT RECORDING COMMITMENT TO CONTINUE AND COOPERATE IN THE STUDY TRACING LOST SUBJECTS, TRYING TO INCLUDE THEM IN THE STUDY CONSIDERING INFORMATION OF LOST PERSONS AT THE TIME OF ANALYSIS KEEPING NON-RESPONSE AT A LOW LEVEL TO IMPROVE THE VALIDITY 10/7/2020 Cohort Studies 16

Examples of Cohort Studies 1. 2. POPULATION-BASED 1. CARDIOVASCULAR 2. CHILD HEALTH 3. SPECIAL EXPOSURES NON-POPULATION BASED 1. OCCUPATIONAL – for convenience 2. OCCUPATIONAL – to study the occupation 3. HEALTH CARE SETTINGS 4. VETERANS 10/7/2020 Cohort Studies 17

CARDIOVASCULAR DISEASE n n n USA: Framingham, MA; Tecumseh, MI; Evans county, GA; Muscatine, IA; Bogalusa, LA (children) WHO MONICA (multi-center) North Karelia, Norway 10/7/2020 Cohort Studies 18

Framingham Study (1951 – present time) n n n 1 ST Step: Selection of cohorts Initially, 5209 subjects were enrolled into the study Currently, thousands of people are followed up, both for cardiovascular risk factors (e. g. high serum cholesterol, smoking, hypertension, BMI, etc) and possible outcomes 10/7/2020 Cohort Studies 19

SPECIAL EXPOSURES n n n Atomic Bomb Casualty Commission (ABCC): Hiroshima and Nagasaki survivors (effects of radiation) Dutch famine survivors (effects of starvation) Seveso (effects of dioxin exposure) 10/7/2020 Cohort Studies 20

OCCUPATION BASED, TO STUDY EXPOSURES n n Benzene-workers (leukemia) Coke-oven workers (lung cancer) Asbestos workers (lung cancer) Radium dial painters (oral cancer) 10/7/2020 Cohort Studies 21

Epidemiological Measures n n n Frequency measures: incidence; prevalence Effect measures: risk ratio (relative risk); odds ratio Impact measures: attributable fraction; prevented fraction 10/7/2020 Cohort Studies 22

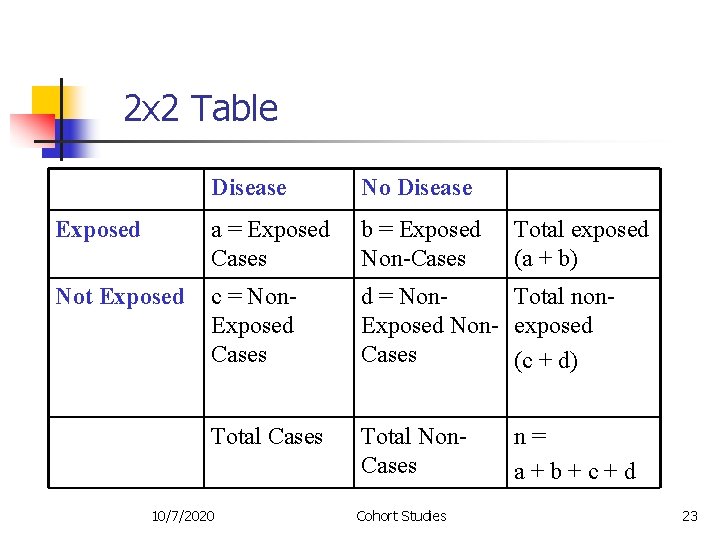
2 x 2 Table Disease No Disease Exposed a = Exposed Cases b = Exposed Non-Cases Not Exposed c = Non. Exposed Cases d = Non. Total non. Exposed Non- exposed Cases (c + d) Total Cases Total Non. Cases 10/7/2020 Cohort Studies Total exposed (a + b) n= a+b+c+d 23

INCIDENCE RATES INCIDENCE AMONG THE EXPOSED (NEW CASES AMONG THE SMOKERS) = (A/ A+B) n INCIDENCE AMONG THE NON-EXPOSED (NEW CASES AMONG THE NON-SMOKERS) = n (C/ C+D ) 10/7/2020 Cohort Studies 24

Risk n n n Calculations Relative Risk or Risk Ratio (RR) = [A / (A+B)] / [C / C +D)] (incidence in the exposed (smokers) / incidence in the nonexposed (non-smokers) Attributable risk (AR = excess risk/ incidence among the exposed (AETIOLOGICAL FRACTION) Population attributable risk (PAR) = incidence in the total population minus incidence among the non-exposed. 10/7/2020 Cohort Studies 25

RR Interpretations n n n Unity: exposure has no effect on outcome in the studied population (cohort) More than 1: exposed have a higher risk of developing the outcome, compared to the unexposed Less than 1: either no relationship, or a “protective” one exists (e. g. effect of interventions, immunization, health education, management, etc) 10/7/2020 Cohort Studies 26

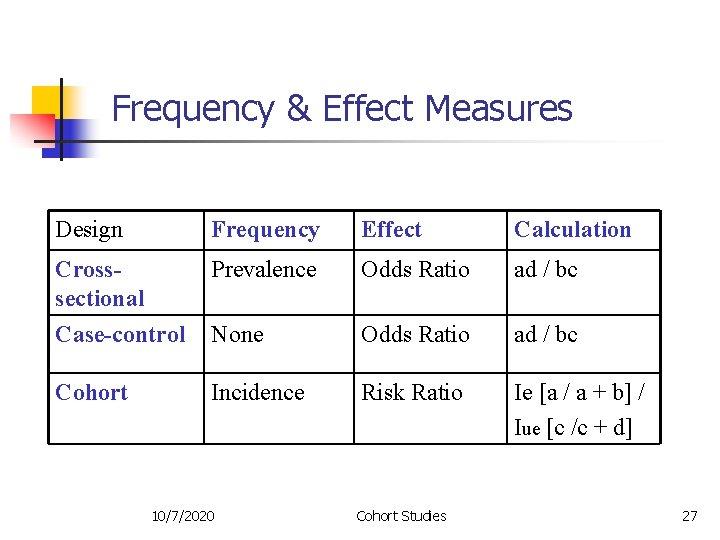
Frequency & Effect Measures Design Frequency Effect Calculation Crosssectional Prevalence Odds Ratio ad / bc Case-control None Odds Ratio ad / bc Cohort Incidence Risk Ratio Ie [a / a + b] / Iue [c /c + d] 10/7/2020 Cohort Studies 27

References 1. 2. 3. 4. 5. Last JM. A dictionary of epidemiology. 5 th edition. New York, Oxford, Toronto: Oxford University Press, 2008. Gordis L. Epidemiology. Fourth edition. Philadelphia, London, New York, Sydney: W. B. Saunders, 2009. Beaglehole R, Bonita R, Kjellstrom T. 2 nd edition. Basic epidemiology. Geneva: WHO, 2006. Friis RH, Sellers TA. Epidemiology for public health practice. Gaithersburg, MD: Aspen Publishers, 2009. Kelsey JL, Thompson WD, Evans AL. Methods in observational epidemiology. New York: Oxford University Press, 1986. 10/7/2020 Cohort Studies 28