Cohesion Collectively hydrogen bonds hold water molecules together
Cohesion • Collectively, hydrogen bonds hold water molecules together, a phenomenon called cohesion • Cohesion helps the transport of water against gravity in plants • Adhesion is an attraction between different substances, for example, between water and plant cell walls Animation: Water Transport 1 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
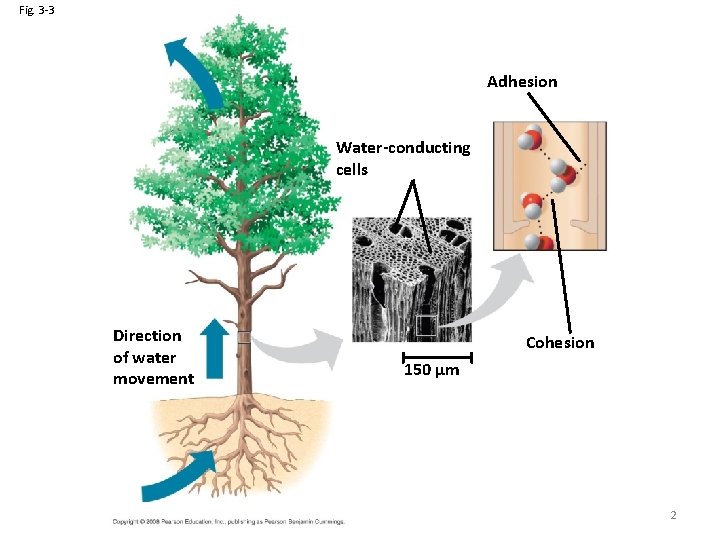
Fig. 3 -3 Adhesion Water-conducting cells Direction of water movement Cohesion 150 µm 2

Universal Solvent • A solution is a liquid that is a homogeneous mixture of substances • A solvent is the dissolving agent of a solution • The solute is the substance that is dissolved • An aqueous solution is one in which water is the solvent 3 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

The Formation of Bonds with Carbon • With four valence electrons, carbon can form four covalent bonds with a variety of atoms • This tetravalence makes large, complex molecules possible • In molecules with multiple carbons, each carbon bonded to four other atoms has a tetrahedral shape • However, when two carbon atoms are joined by a double bond, the molecule has a flat shape 5 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
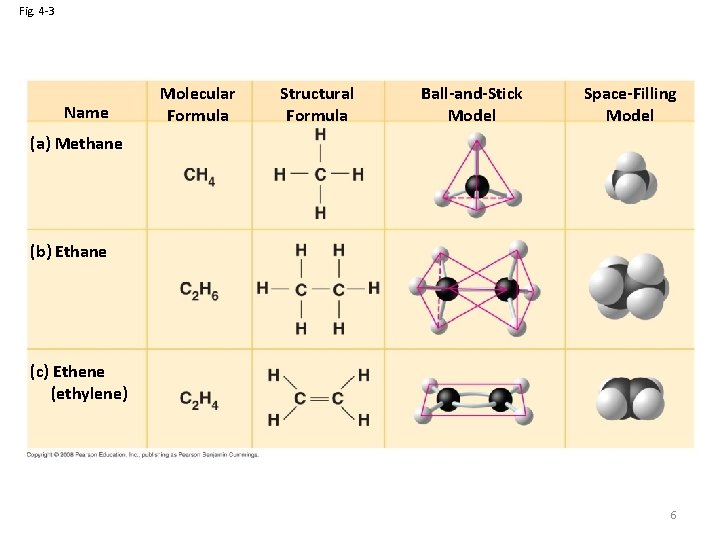
Fig. 4 -3 Name Molecular Formula Structural Formula Ball-and-Stick Model Space-Filling Model (a) Methane (b) Ethane (c) Ethene (ethylene) 6
ATP: An Important Source of Energy for Cellular Processes • One phosphate molecule, adenosine triphosphate (ATP), is the primary energytransferring molecule in the cell • ATP consists of an organic molecule called adenosine attached to a string of three phosphate groups 7 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
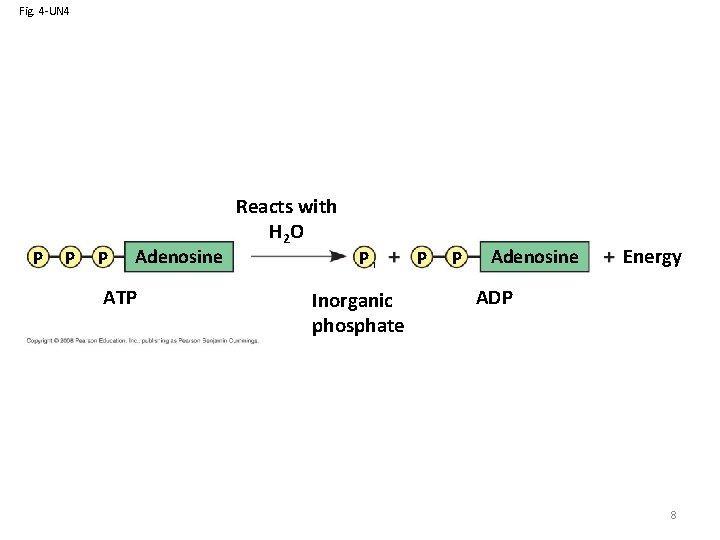
Fig. 4 -UN 4 P P P Adenosine ATP Reacts with H 2 O Pi Inorganic phosphate P P Adenosine Energy ADP 8
Carbohydrates serve as fuel and building material • Carbohydrates include sugars and the polymers of sugars • The simplest carbohydrates are monosaccharides, or single sugars • Carbohydrate macromolecules are polysaccharides, polymers composed of many sugar building blocks 9 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

• Starch Plants store surplus starch as granules within chloroplasts and other plastids • Cellulose is a major component of the tough wall of plant cells • Chitin provides structural support for the cell walls of many fungi 10 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Concept 5. 3: Lipids are a diverse group of hydrophobic molecules • Lipids store energy and maintain membranes • Lipids are hydrophobic because they consist mostly of hydrocarbons, which form nonpolar covalent bonds 11 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

• Saturated fatty acids have the maximum number of hydrogen atoms possible and no double bonds • Unsaturated fatty acids have one or more double bonds Animation: Fats 12 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
The Roles of Nucleic Acids • There are two types of nucleic acids: –Deoxyribonucleic acid (DNA) directs synthesis of messenger RNA –Ribonucleic acid (RNA) controls protein synthesis 13 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
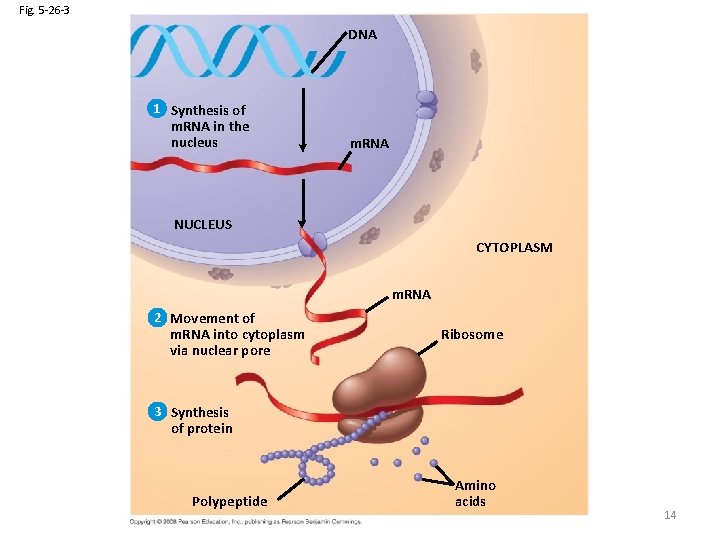
Fig. 5 -26 -3 DNA 1 Synthesis of m. RNA in the nucleus m. RNA NUCLEUS CYTOPLASM m. RNA 2 Movement of m. RNA into cytoplasm via nuclear pore Ribosome 3 Synthesis of protein Polypeptide Amino acids 14
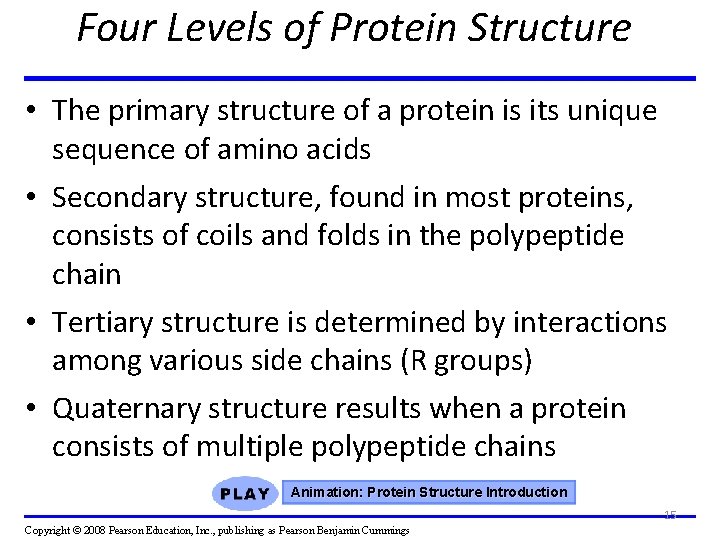
Four Levels of Protein Structure • The primary structure of a protein is its unique sequence of amino acids • Secondary structure, found in most proteins, consists of coils and folds in the polypeptide chain • Tertiary structure is determined by interactions among various side chains (R groups) • Quaternary structure results when a protein consists of multiple polypeptide chains Animation: Protein Structure Introduction 15 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
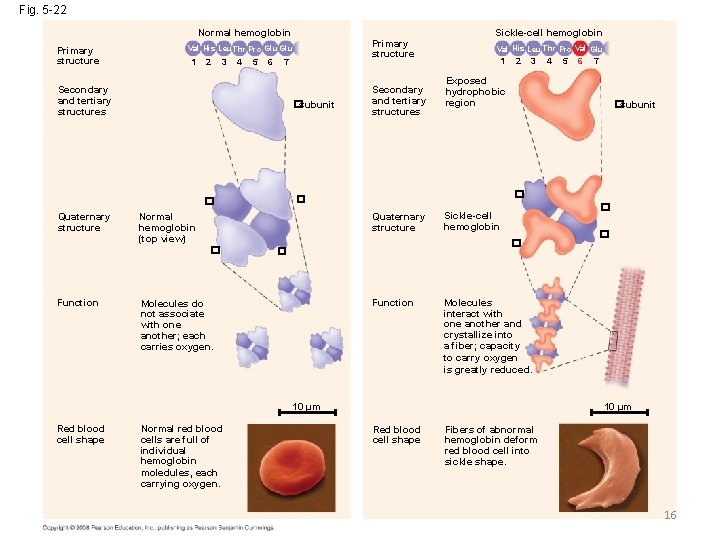
Fig. 5 -22 Normal hemoglobin Primary structure 1 2 3 4 5 6 7 Secondary and tertiary structures �subunit Normal hemoglobin (top view) Val His Leu Thr Pro Val Glu 1 2 3 Normal red blood cells are full of individual hemoglobin moledules, each carrying oxygen. 6 7 �subunit � Sickle-cell hemoglobin � Function � Molecules interact with one another and crystallize into a fiber; capacity to carry oxygen is greatly reduced. 10 µm Red blood cell shape 5 Exposed hydrophobic region � Molecules do not associate with one another; each carries oxygen. 4 � Quaternary structure � Function Secondary and tertiary structures Sickle-cell hemoglobin � � Quaternary structure Primary structure Val His Leu Thr Pro Glu 10 µm Red blood cell shape Fibers of abnormal hemoglobin deform red blood cell into sickle shape. 16
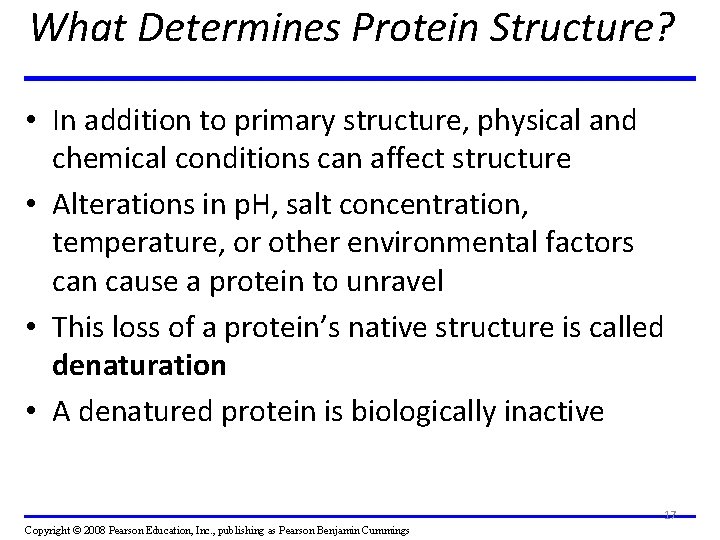
What Determines Protein Structure? • In addition to primary structure, physical and chemical conditions can affect structure • Alterations in p. H, salt concentration, temperature, or other environmental factors can cause a protein to unravel • This loss of a protein’s native structure is called denaturation • A denatured protein is biologically inactive 17 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
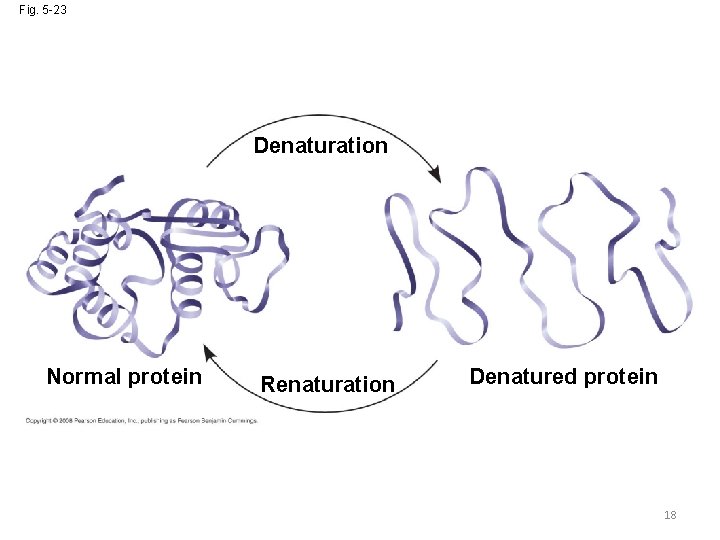
Fig. 5 -23 Denaturation Normal protein Renaturation Denatured protein 18
PPT panels I used • Unit 1 • C 5 10&33, 35&41, 75&89, 93&94, 104&107 • C 6 22&23, 24&30, 40&41 C 7 23&24, 39&42, 47&61 C 8 22&ice cube, 44&47, 54&57 19
- Slides: 19