Coffee Cup Calorimetry Lab Lesson Eight Bond Lengths

Coffee Cup Calorimetry Lab Lesson Eight
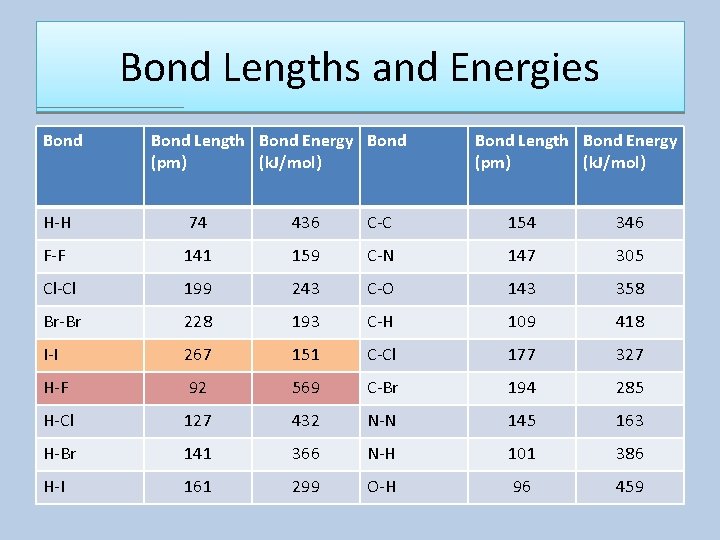
Bond Lengths and Energies Bond Length Bond Energy Bond (pm) (k. J/mol) Bond Length Bond Energy (pm) (k. J/mol) H-H 74 436 C-C 154 346 F-F 141 159 C-N 147 305 Cl-Cl 199 243 C-O 143 358 Br-Br 228 193 C-H 109 418 I-I 267 151 C-Cl 177 327 H-F 92 569 C-Br 194 285 H-Cl 127 432 N-N 145 163 H-Br 141 366 N-H 101 386 H-I 161 299 O-H 96 459
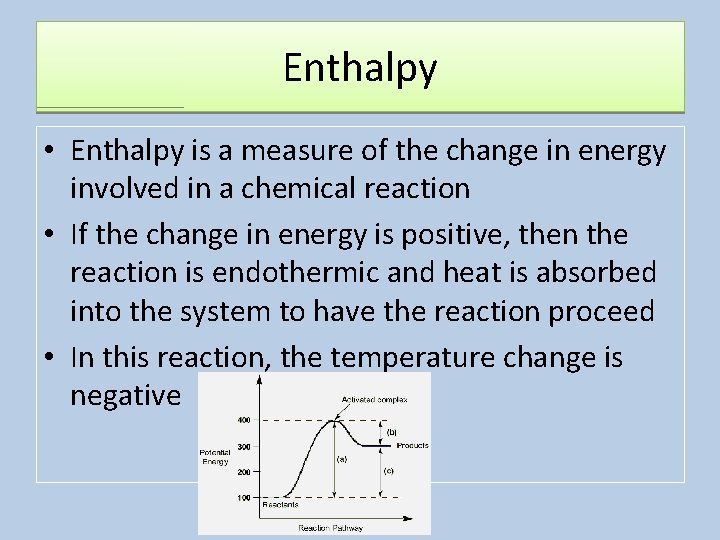
Enthalpy • Enthalpy is a measure of the change in energy involved in a chemical reaction • If the change in energy is positive, then the reaction is endothermic and heat is absorbed into the system to have the reaction proceed • In this reaction, the temperature change is negative
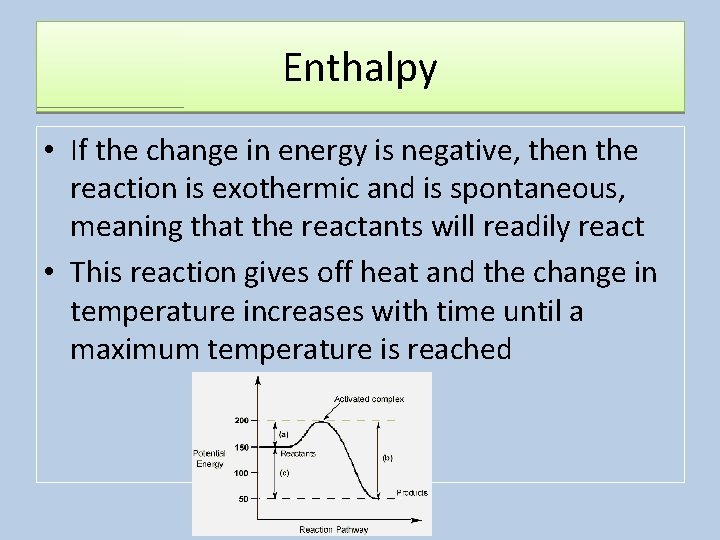
Enthalpy • If the change in energy is negative, then the reaction is exothermic and is spontaneous, meaning that the reactants will readily react • This reaction gives off heat and the change in temperature increases with time until a maximum temperature is reached

Gummy Bear Experiment • Is this reaction endothermic or exothermic? How do you know? • http: //video. google. com/videoplay? docid=74334 92777550198070 • How does this experiment relate to the bond breaking and forming that we’ve discussed? • http: //www. youtube. com/watch? v=e. Ck 0 l. YB_8 c 0 • Alkali metals + Water
- Slides: 5