COAPT A Randomized Trial of Transcatheter Mitral Valve




- Slides: 9

COAPT A Randomized Trial of Transcatheter Mitral Valve Leaflet Approximation in Patients with Heart Failure and Secondary Mitral Regurgitation Michael Mack William T. Abraham Jo. Ann Lindenfeld Gregg W. Stone On behalf of the COAPT Investigators COAPT (NCT 01626079)

Disclosure Statement • • Abbott – Co-PI COAPT Trial Edwards Lifesciences- Co-PI PARTNER 3 Trial Medtronic- Study Chair-Apollo Trial Gore- Consultant The COAPT (NCT 01626079) study was sponsored by Abbott

The COAPT Trial Cardiovascular Outcomes Assessment of the Mitra. Clip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation A parallel-controlled, open-label, multicenter trial in 614 patients with heart failure and moderate-to-severe (3+) or severe (4+) secondary MR who remained symptomatic despite maximally-tolerated GDMT Randomize 1: 1* Mitra. Clip + GDMT alone N=312 N=302 Follow-up at 30 d, 6 mo, 1 y, 18 mo, 2 y, 3 y, 4 y, 5 y *Stratified by cardiomyopathy etiology (ischemic vs. non-ischemic) and site

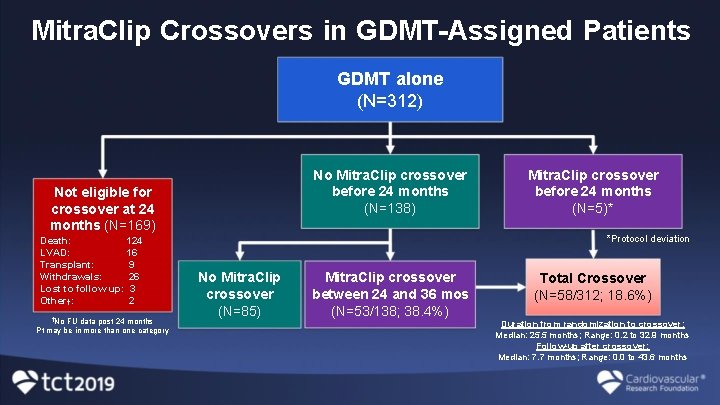
Mitra. Clip Crossovers in GDMT-Assigned Patients GDMT alone (N=312) No Mitra. Clip crossover before 24 months (N=138) Not eligible for crossover at 24 months (N=169) 124 Death: 16 LVAD: 9 Transplant: Withdrawals: 26 Lost to follow up: 3 Other†: 2 †No FU data post 24 months Pt may be in more than one category Mitra. Clip crossover before 24 months (N=5)* *Protocol deviation No Mitra. Clip crossover (N=85) Mitra. Clip crossover between 24 and 36 mos (N=53/138; 38. 4%) Total Crossover (N=58/312; 18. 6%) Duration from randomization to crossover: Median: 25. 5 months; Range: 0. 2 to 32. 9 months Follow-up after crossover: Median: 7. 7 months; Range: 0. 0 to 43. 6 months

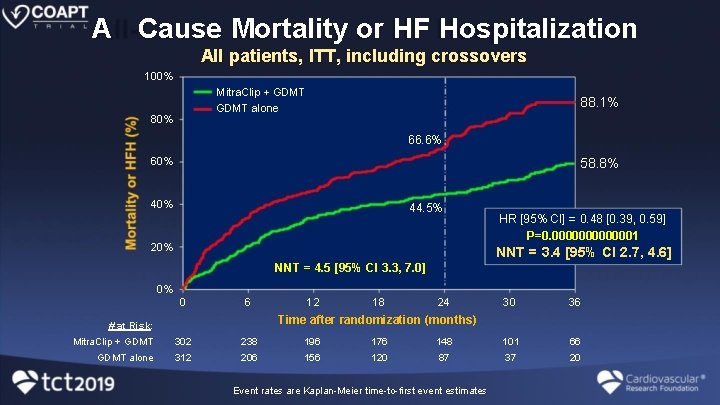
A Cause Mortality or HF Hospitalization All patients, ITT, including crossovers 100% Mitra. Clip + GDMT 88. 1% GDMT alone 80% 66. 6% 60% 58. 8% 40% 44. 5% 20% HR [95% CI] = 0. 48 [0. 39, 0. 59] P=0. 0000001 NNT = 3. 4 [95% CI 2. 7, 4. 6] NNT = 4. 5 [95% CI 3. 3, 7. 0] 0% 0 6 12 18 24 30 36 Time after randomization (months) # at Risk: Mitra. Clip + GDMT 302 238 196 176 148 101 66 GDMT alone 312 206 156 120 87 37 20 Event rates are Kaplan-Meier time-to-first event estimates

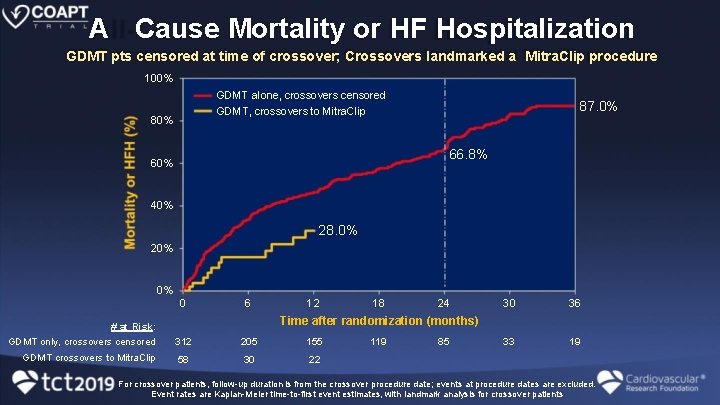
A Cause Mortality or HF Hospitalization GDMT pts censored at time of crossover; Crossovers landmarked a Mitra. Clip procedure 100% GDMT alone, crossovers censored GDMT, crossovers to Mitra. Clip 80% 87. 0% 66. 8% 60% 40% 28. 0% 20% 0% 0 6 12 18 24 30 36 33 19 Time after randomization (months) # at Risk: GDMT only, crossovers censored 312 205 155 GDMT crossovers to Mitra. Clip 58 30 22 119 85 For crossover patients, follow-up duration is from the crossover procedure date; events at procedure dates are excluded. Event rates are Kaplan-Meier time-to-first event estimates, with landmark analysis for crossover patients

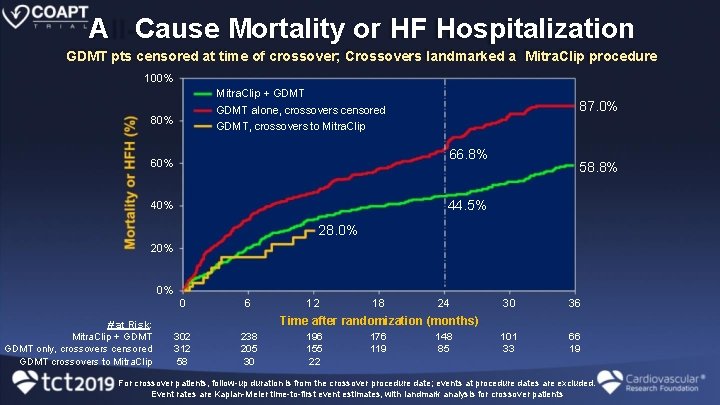
A Cause Mortality or HF Hospitalization GDMT pts censored at time of crossover; Crossovers landmarked a Mitra. Clip procedure 100% Mitra. Clip + GDMT 87. 0% GDMT alone, crossovers censored GDMT, crossovers to Mitra. Clip 80% 66. 8% 60% 58. 8% 44. 5% 40% 28. 0% 20% 0% # at Risk: Mitra. Clip + GDMT only, crossovers censored GDMT crossovers to Mitra. Clip 0 6 302 312 58 238 205 30 12 18 24 30 36 101 33 66 19 Time after randomization (months) 196 155 22 176 119 148 85 For crossover patients, follow-up duration is from the crossover procedure date; events at procedure dates are excluded. Event rates are Kaplan-Meier time-to-first event estimates, with landmark analysis for crossover patients

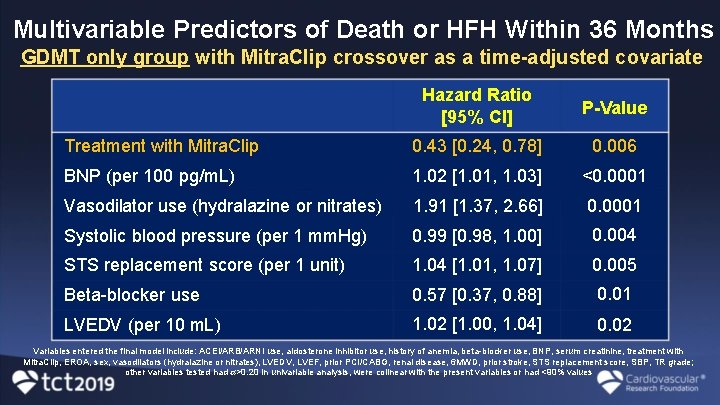
Multivariable Predictors of Death or HFH Within 36 Months GDMT only group with Mitra. Clip crossover as a time-adjusted covariate Hazard Ratio [95% CI] P-Value Treatment with Mitra. Clip 0. 43 [0. 24, 0. 78] 0. 006 BNP (per 100 pg/m. L) 1. 02 [1. 01, 1. 03] <0. 0001 Vasodilator use (hydralazine or nitrates) 1. 91 [1. 37, 2. 66] 0. 0001 Systolic blood pressure (per 1 mm. Hg) 0. 99 [0. 98, 1. 00] 0. 004 STS replacement score (per 1 unit) 1. 04 [1. 01, 1. 07] 0. 005 Beta-blocker use 0. 57 [0. 37, 0. 88] 0. 01 LVEDV (per 10 m. L) 1. 02 [1. 00, 1. 04] 0. 02 Variables entered the final model include: ACEi/ARB/ARNI use, aldosterone inhibitor use, history of anemia, beta-blocker use, BNP, serum creatinine, treatment with Mitra. Clip, EROA, sex, vasodilators (hydralazine or nitrates), LVEDV, LVEF, prior PCI/CABG, renal disease, 6 MWD, prior stroke, STS replacement score, SBP, TR grade; other variables tested had >0. 20 in univariable analysis, were colinear with the present variables or had <90% values

Conclusions In pts with HF and 3+/4+ secondary MR who remained symptomatic despite maximally-tolerated GDMT: • At 36 months transcatheter mitral leaflet approximation with the Mitra. Clip was safe, provided durable reduction in MR, reduced the rate of HF hospitalizations, and improved survival, QOL and functional capacity compared to GDMT alone • GDMT only-assigned pts who crossed-over and received a Mitra. Clip experienced fewer HF hospitalizations and deaths or HFHs within 12 months than those who did not crossover, with rates comparable to pts originally assigned to the Mitra. Clip