CO 2 Foam Mobility Control at Reservoir Conditions

CO 2 Foam Mobility Control at Reservoir Conditions: High Temperature, High Salinity and Carbonate Reservoirs Presented by Leyu Cui 1 George Hirasaki 1, Yunshen Chen 2, Amro Elhag 2, Ahmed A. Abdala 3, Lucas J. Lu 1, 3, Maura Puerto 1, Kun Ma 1*, Ivan Tanakov 1, Ramesh Pudasaini 1, Keith P. Johnston 2, and Sibani L. Biswal 1 1 Rice University; 2 University of Texas at Austin; 3 the Petroleum Institute at Abu Dhabi; *currently affiliation is TOTAL Consortium Meeting in Rice, April. 2014 Sponsored by ADNOC and PI 1
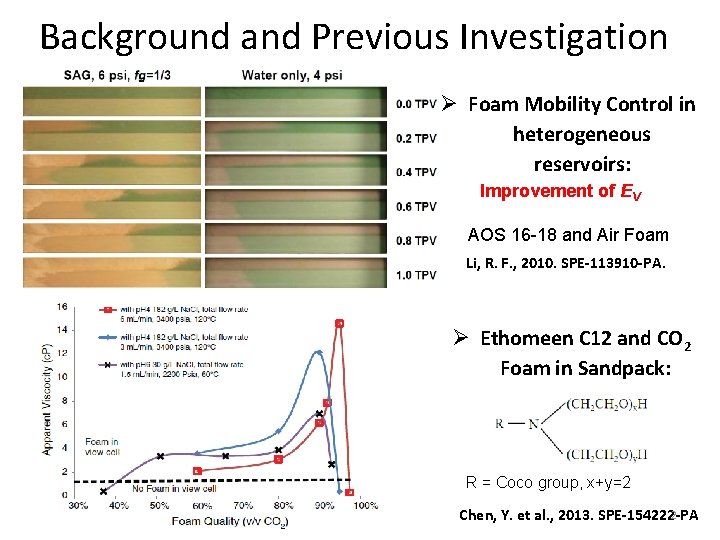
Background and Previous Investigation Ø Foam Mobility Control in heterogeneous reservoirs: Improvement of EV AOS 16 -18 and Air Foam Li, R. F. , 2010. SPE-113910 -PA. Ø Ethomeen C 12 and CO 2 Foam in Sandpack: R = Coco group, x+y=2 2 Chen, Y. et al. , 2013. SPE-154222 -PA
Evaluation Procedure of Surfactant Formulations for Foam EOR Can CO 2 foam be generated and applied at reservoir conditions for mobility control? A systematic procedure should be used to evaluate the foam process: Ø Evaluation of Surfactant Properties: 1. Solubility 2. Thermal Stability 3. Adsorption 4. *Partitioning Coefficient for CO -soluble surfactant; **Interfacial 2 tension (IFT) for immiscible foam Ø Investigation of Foam Mobility Control 1. *Pre-Screening of Foaming Agents in Sandpack 2. Foam Flooding at Reservoir Conditions *Chen, Y. et al. , 2013. SPE-154222 -PA **Wang, et al. , 2001. SPE-72147 3
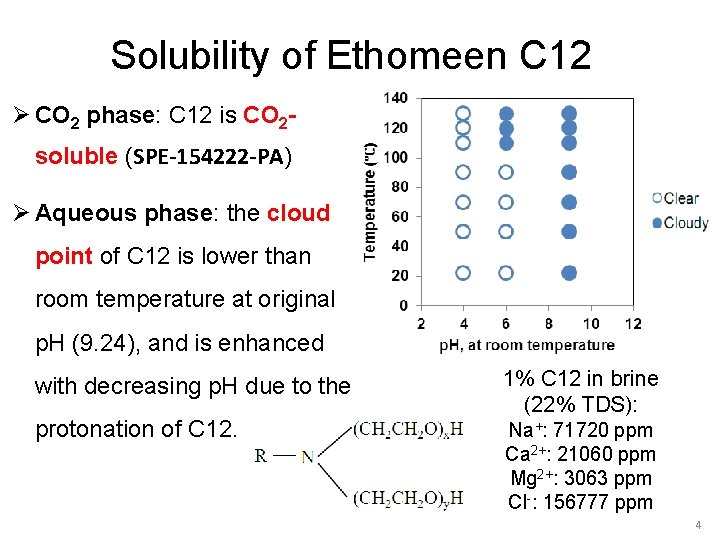
Solubility of Ethomeen C 12 Ø CO 2 phase: C 12 is CO 2 soluble (SPE-154222 -PA) Ø Aqueous phase: the cloud point of C 12 is lower than room temperature at original p. H (9. 24), and is enhanced with decreasing p. H due to the protonation of C 12. 1% C 12 in brine (22% TDS): Na+: 71720 ppm Ca 2+: 21060 ppm Mg 2+: 3063 ppm Cl-: 156777 ppm 4
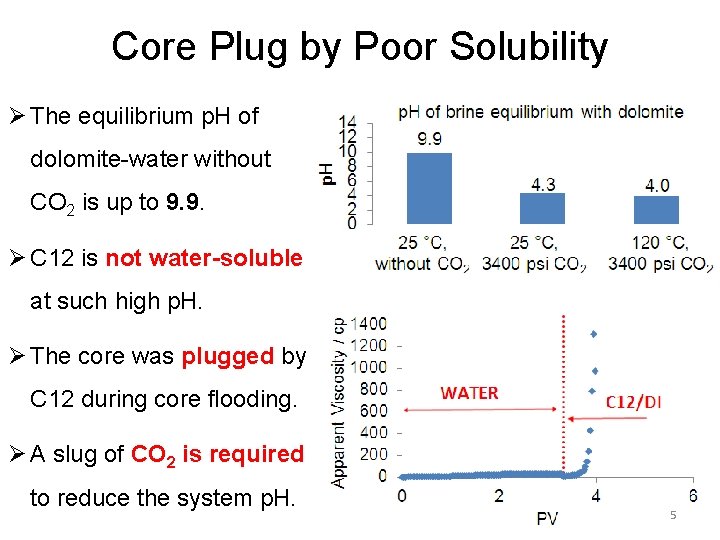
Core Plug by Poor Solubility Ø The equilibrium p. H of dolomite-water without CO 2 is up to 9. 9. Ø C 12 is not water-soluble at such high p. H. Ø The core was plugged by C 12 during core flooding. Ø A slug of CO 2 is required to reduce the system p. H. 5
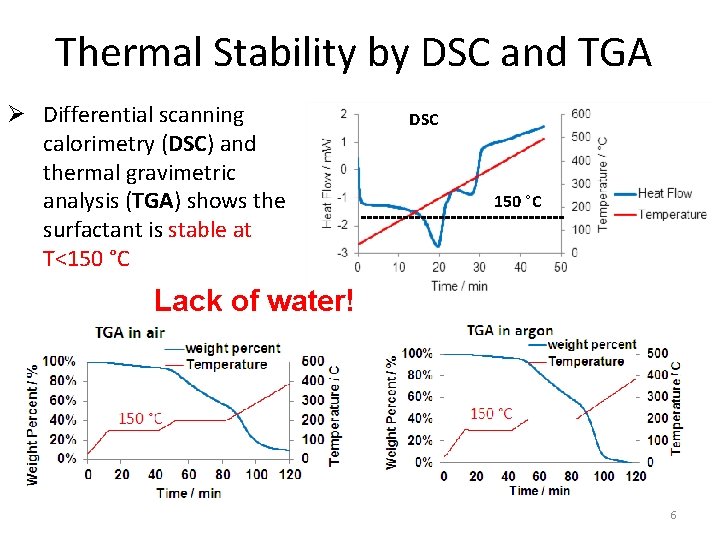
Thermal Stability by DSC and TGA Ø Differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA) shows the surfactant is stable at T<150 °C DSC 150 °C Lack of water! 6
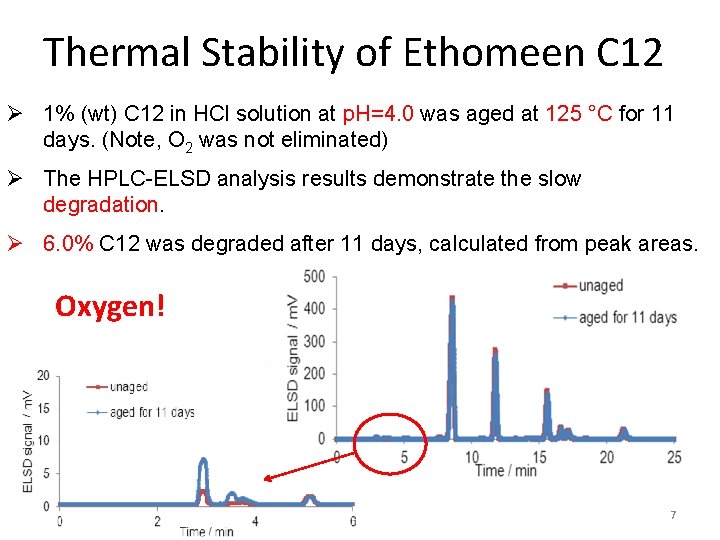
Thermal Stability of Ethomeen C 12 Ø 1% (wt) C 12 in HCl solution at p. H=4. 0 was aged at 125 °C for 11 days. (Note, O 2 was not eliminated) Ø The HPLC-ELSD analysis results demonstrate the slow degradation. Ø 6. 0% C 12 was degraded after 11 days, calculated from peak areas. Oxygen! 7
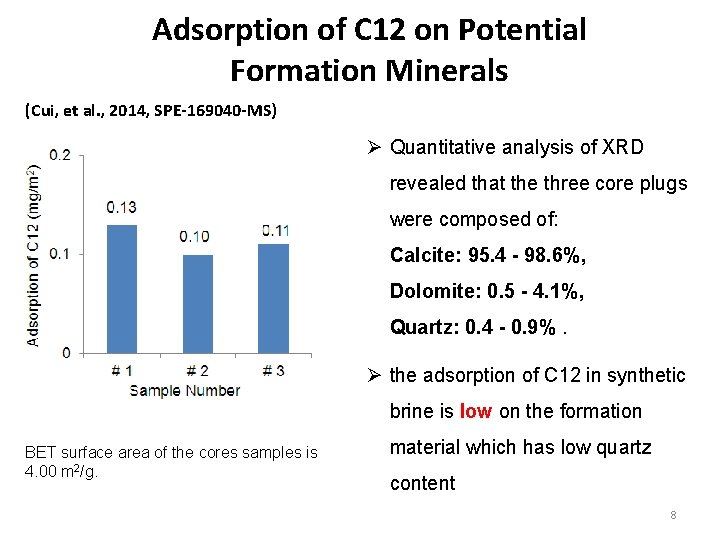
Adsorption of C 12 on Potential Formation Minerals (Cui, et al. , 2014, SPE-169040 -MS) Ø Quantitative analysis of XRD revealed that the three core plugs were composed of: Calcite: 95. 4 - 98. 6%, Dolomite: 0. 5 - 4. 1%, Quartz: 0. 4 - 0. 9%. Ø the adsorption of C 12 in synthetic brine is low on the formation BET surface area of the cores samples is 4. 00 m 2/g. material which has low quartz content 8
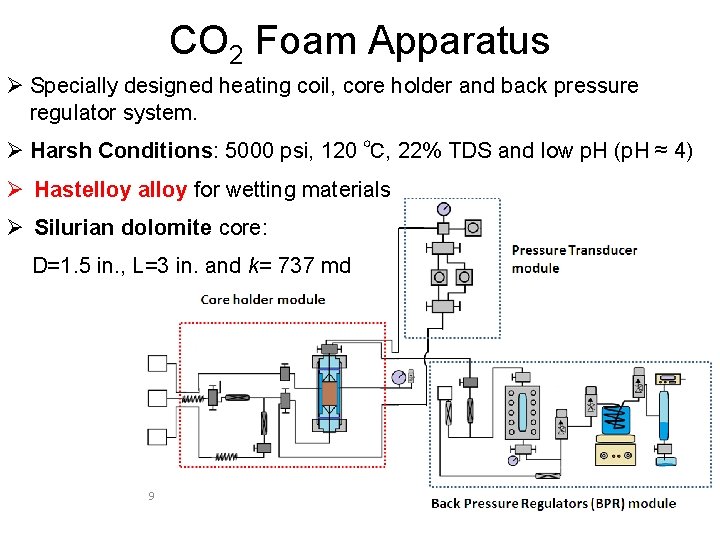
CO 2 Foam Apparatus Ø Specially designed heating coil, core holder and back pressure regulator system. Ø Harsh Conditions: 5000 psi, 120 ℃, 22% TDS and low p. H (p. H ≈ 4) Ø Hastelloy alloy for wetting materials Ø Silurian dolomite core: D=1. 5 in. , L=3 in. and k= 737 md 9
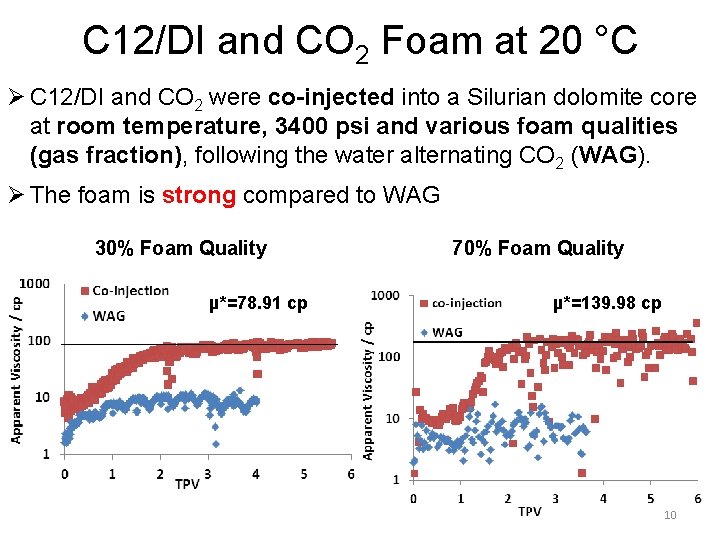
C 12/DI and CO 2 Foam at 20 °C Ø C 12/DI and CO 2 were co-injected into a Silurian dolomite core at room temperature, 3400 psi and various foam qualities (gas fraction), following the water alternating CO 2 (WAG). Ø The foam is strong compared to WAG 30% Foam Quality µ*=78. 91 cp 70% Foam Quality µ*=139. 98 cp 10
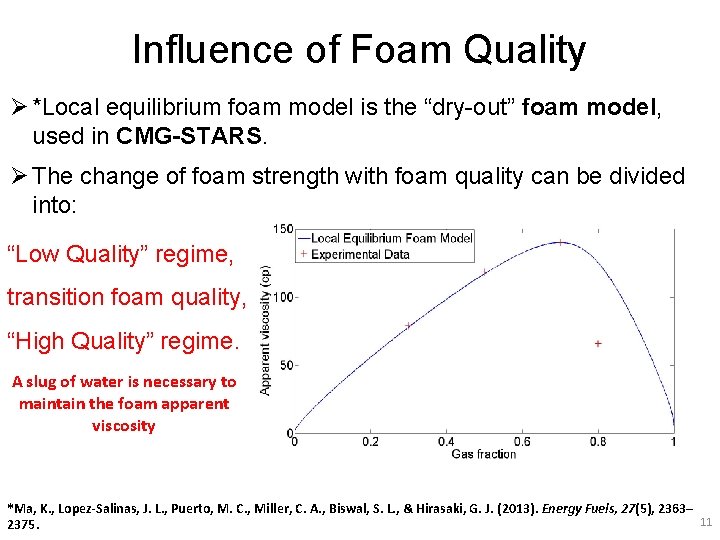
Influence of Foam Quality Ø *Local equilibrium foam model is the “dry-out” foam model, used in CMG-STARS. Ø The change of foam strength with foam quality can be divided into: “Low Quality” regime, transition foam quality, “High Quality” regime. A slug of water is necessary to maintain the foam apparent viscosity *Ma, K. , Lopez-Salinas, J. L. , Puerto, M. C. , Miller, C. A. , Biswal, S. L. , & Hirasaki, G. J. (2013). Energy Fuels, 27(5), 2363– 11 2375.
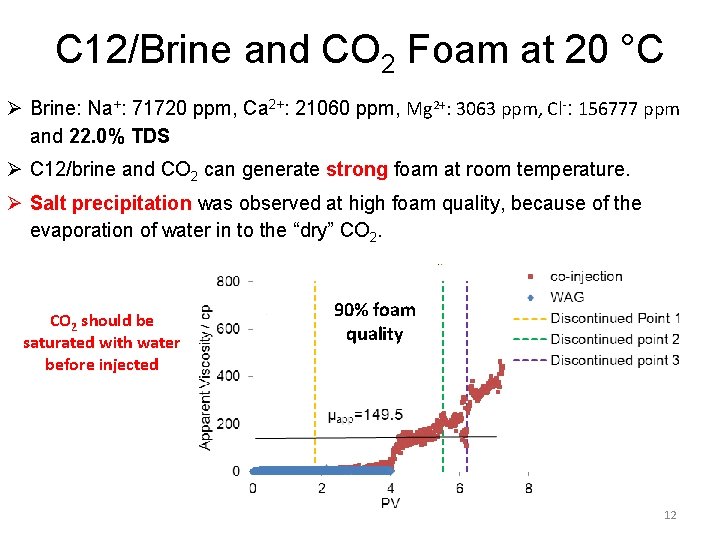
C 12/Brine and CO 2 Foam at 20 °C Ø Brine: Na+: 71720 ppm, Ca 2+: 21060 ppm, Mg 2+: 3063 ppm, Cl-: 156777 ppm and 22. 0% TDS Ø C 12/brine and CO 2 can generate strong foam at room temperature. Ø Salt precipitation was observed at high foam quality, because of the evaporation of water in to the “dry” CO 2 should be saturated with water before injected 90% foam quality 12
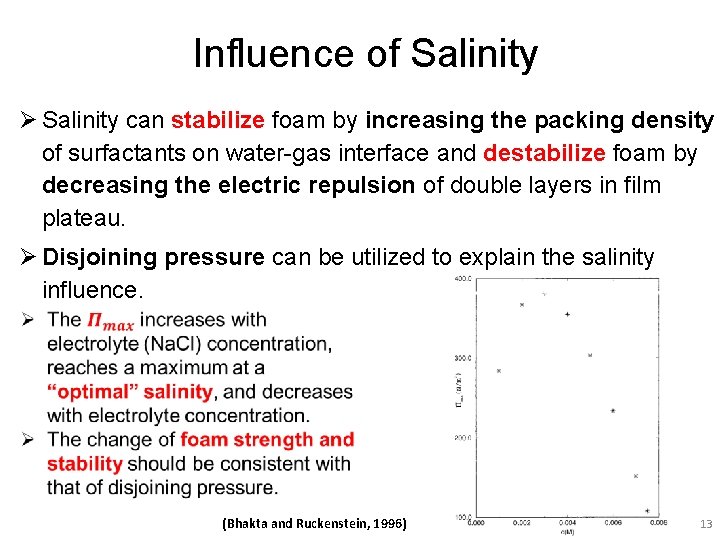
Influence of Salinity Ø Salinity can stabilize foam by increasing the packing density of surfactants on water-gas interface and destabilize foam by decreasing the electric repulsion of double layers in film plateau. Ø Disjoining pressure can be utilized to explain the salinity influence. (Bhakta and Ruckenstein, 1996) 13
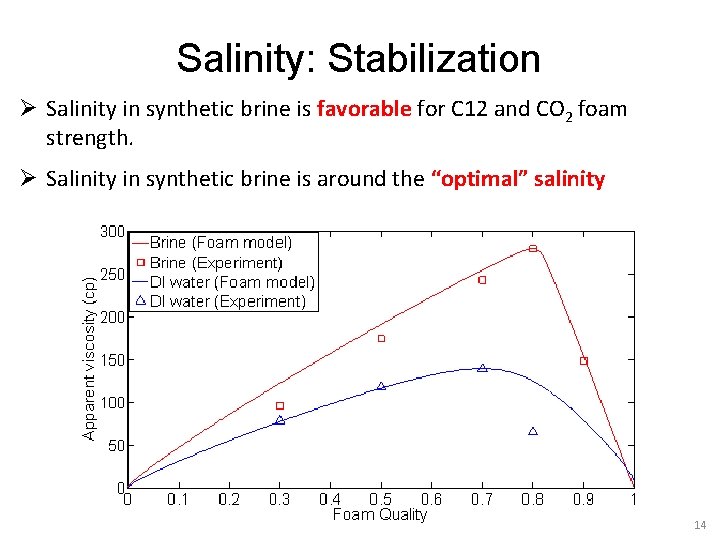
Salinity: Stabilization Ø Salinity in synthetic brine is favorable for C 12 and CO 2 foam strength. Ø Salinity in synthetic brine is around the “optimal” salinity 14
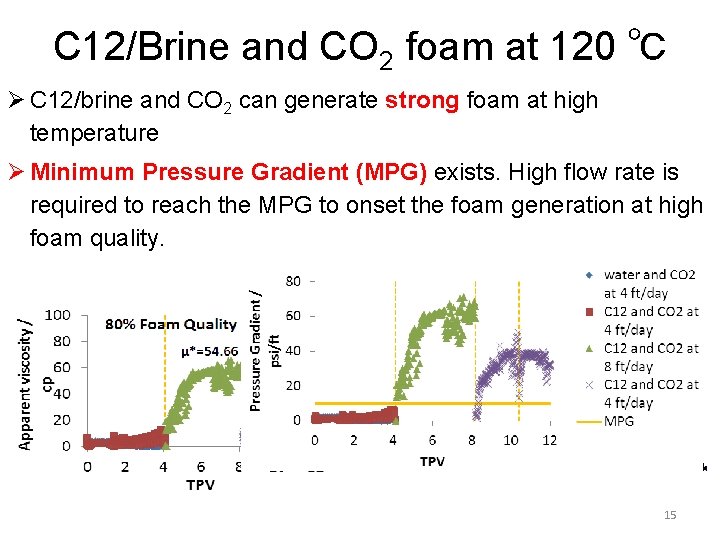
C 12/Brine and CO 2 foam at 120 ℃ Ø C 12/brine and CO 2 can generate strong foam at high temperature Ø Minimum Pressure Gradient (MPG) exists. High flow rate is required to reach the MPG to onset the foam generation at high foam quality. 15
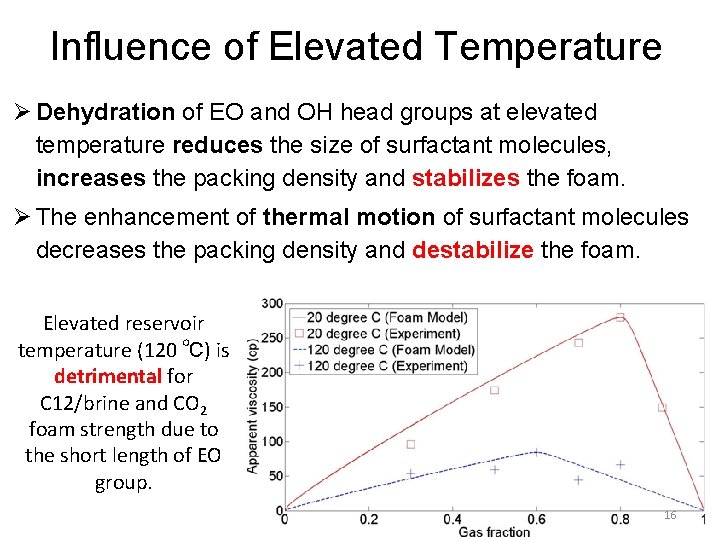
Influence of Elevated Temperature Ø Dehydration of EO and OH head groups at elevated temperature reduces the size of surfactant molecules, increases the packing density and stabilizes the foam. Ø The enhancement of thermal motion of surfactant molecules decreases the packing density and destabilize the foam. Elevated reservoir temperature (120 ℃) is detrimental for C 12/brine and CO 2 foam strength due to the short length of EO group. 16
Conclusions – Evaluation Results Ø The solubility of C 12 depends on p. H and temperature. C 12 is water -soluble at 120 °C in CO 2 flooding processes. Ø C 12 is slowly degraded at 125 °C and p. H=4. But oxygen was not eliminated and may cause this degradation. Ø The adsorption of C 12 is low on relative pure carbonate surface. Ø Ethomeen C 12 and CO 2 can generate strong foam at reservoir conditions, i. e. , high temperature, high salinity and carbonate minerals. 17

Conclusions – Field Application Ø Ethomeen C 12 is suggested to be injected in CO 2 phase to maintain the solubility at reservoir conditions, because of the low p. H of aqueous phase in the presence of CO 2. Ø A slug of water should be injected to maintain the CO 2 foam strength, although Ethomeen C 12 is a CO 2 -soluble surfactant. Ø The CO 2 phase should be saturated with water before injected to prevent the salt precipitation. Ø The high minimum pressure gradient (10 psi/ft) for foam generation at reservoir conditions may reduce of the injectivity and result in the failure of foam generation in situ. Ø Sufficient divalent cations are needed to suppress the dissolution of carbonate mineral in CO 2 and water flooding. 18

Acknowledgement and Questions? • Thank you. We acknowledge financial support from the Abu Dhabi National Oil Company (ADNOC), and the Petroleum Institute (PI), U. A. E and partial support from the US Department of Energy (under Award No. DE-FE 0005902) 19

Backup 20
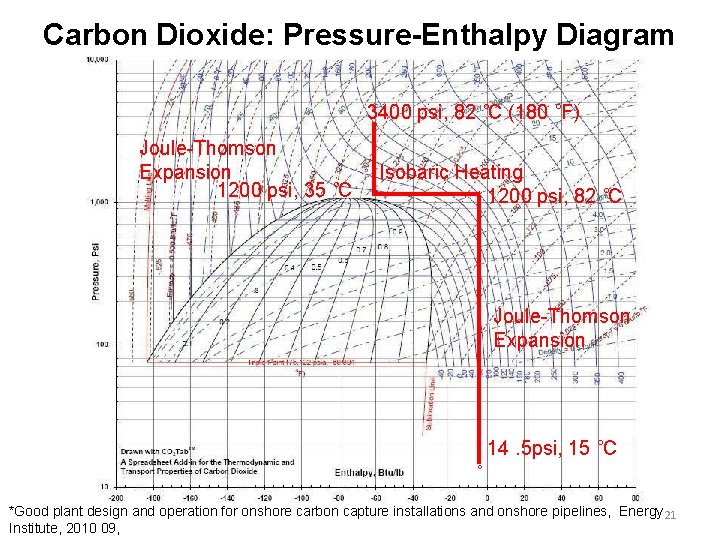
Carbon Dioxide: Pressure-Enthalpy Diagram 3400 psi, 82 ˚C (180 ˚F) Joule-Thomson Expansion 1200 psi, 35 ˚C Isobaric Heating 1200 psi, 82 ˚C Joule-Thomson Expansion 14. 5 psi, 15 ˚C *Good plant design and operation for onshore carbon capture installations and onshore pipelines, Energy 21 Institute, 2010 09,
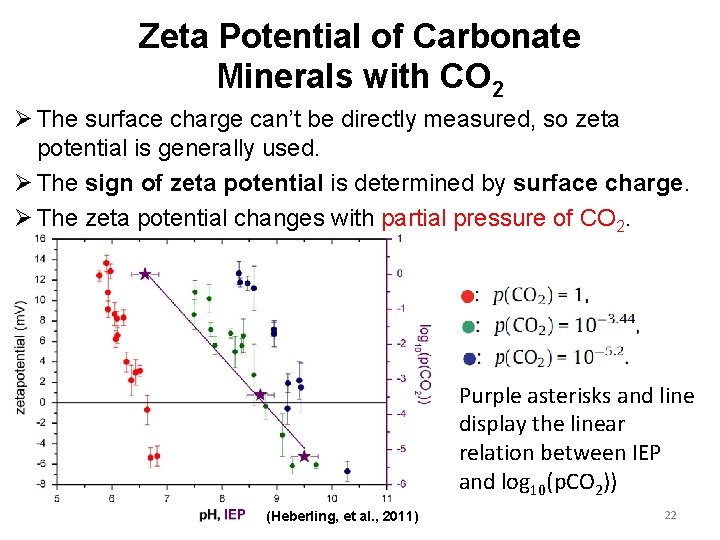
Zeta Potential of Carbonate Minerals with CO 2 Ø The surface charge can’t be directly measured, so zeta potential is generally used. Ø The sign of zeta potential is determined by surface charge. Ø The zeta potential changes with partial pressure of CO 2. Purple asterisks and line display the linear relation between IEP and log 10(p. CO 2)) (Heberling, et al. , 2011) 22
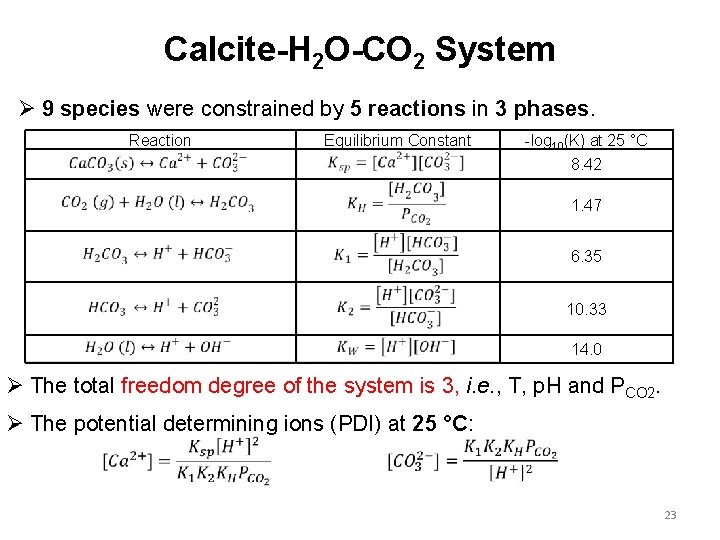
Calcite-H 2 O-CO 2 System Ø 9 species were constrained by 5 reactions in 3 phases. Reaction Equilibrium Constant -log 10(K) at 25 °C 8. 42 1. 47 6. 35 10. 33 14. 0 Ø The total freedom degree of the system is 3, i. e. , T, p. H and PCO 2. Ø The potential determining ions (PDI) at 25 °C: 23
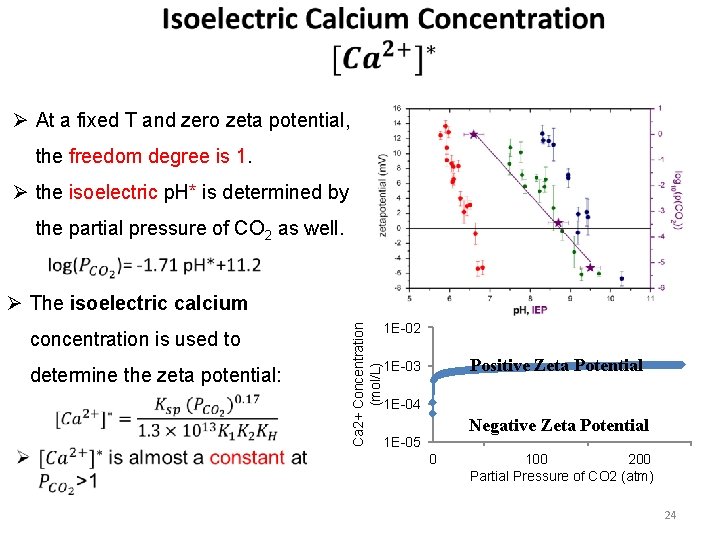
Ø At a fixed T and zero zeta potential, the freedom degree is 1. Ø the isoelectric p. H* is determined by the partial pressure of CO 2 as well. concentration is used to determine the zeta potential: Ca 2+ Concentration (mol/L) Ø The isoelectric calcium 1 E-02 Positive Zeta Potential 1 E-03 1 E-04 Negative Zeta Potential 1 E-05 0 100 200 Partial Pressure of CO 2 (atm) 24
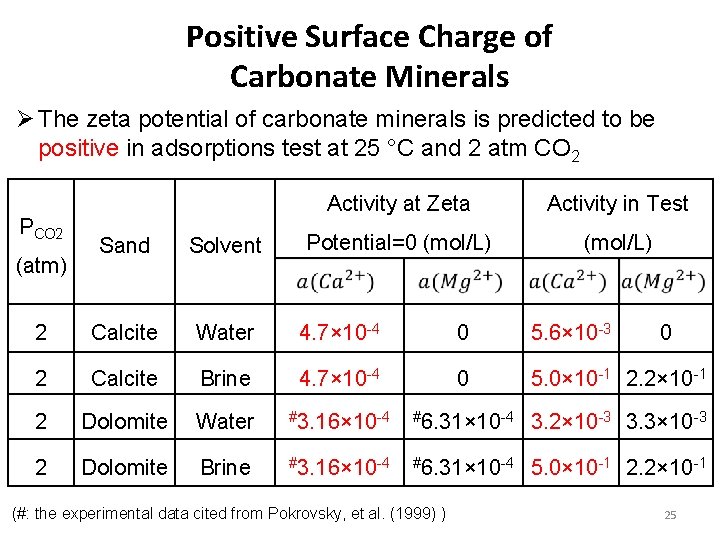
Positive Surface Charge of Carbonate Minerals Ø The zeta potential of carbonate minerals is predicted to be positive in adsorptions test at 25 °C and 2 atm CO 2 PCO 2 Activity at Zeta Activity in Test Potential=0 (mol/L) Sand Solvent 2 Calcite Water 4. 7× 10 -4 0 5. 6× 10 -3 2 Calcite Brine 4. 7× 10 -4 0 5. 0× 10 -1 2. 2× 10 -1 2 Dolomite Water #3. 16× 10 -4 #6. 31× 10 -4 3. 2× 10 -3 3. 3× 10 -3 2 Dolomite Brine #3. 16× 10 -4 #6. 31× 10 -4 5. 0× 10 -1 2. 2× 10 -1 (atm) (#: the experimental data cited from Pokrovsky, et al. (1999) ) 0 25
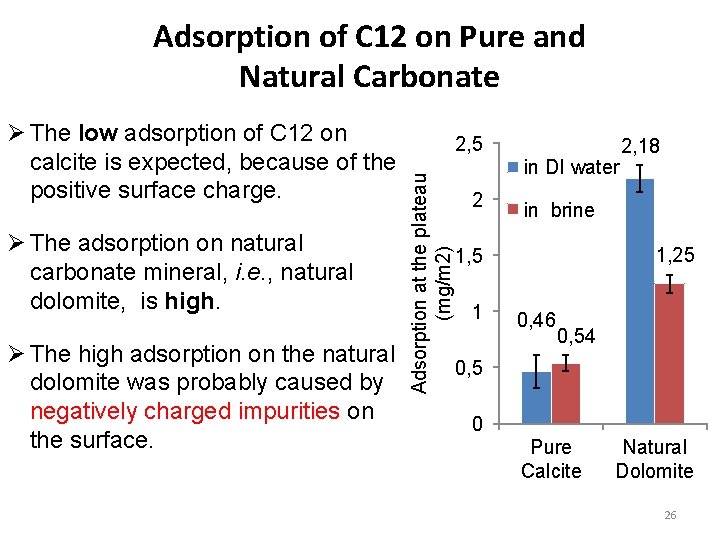
Adsorption of C 12 on Pure and Natural Carbonate Ø The adsorption on natural carbonate mineral, i. e. , natural dolomite, is high. Ø The high adsorption on the natural dolomite was probably caused by negatively charged impurities on the surface. 2, 5 Adsorption at the plateau (mg/m 2) Ø The low adsorption of C 12 on calcite is expected, because of the positive surface charge. in DI water 2 in brine 1, 25 1, 5 1 2, 18 0, 46 0, 54 0, 5 0 Pure Calcite Natural Dolomite 26
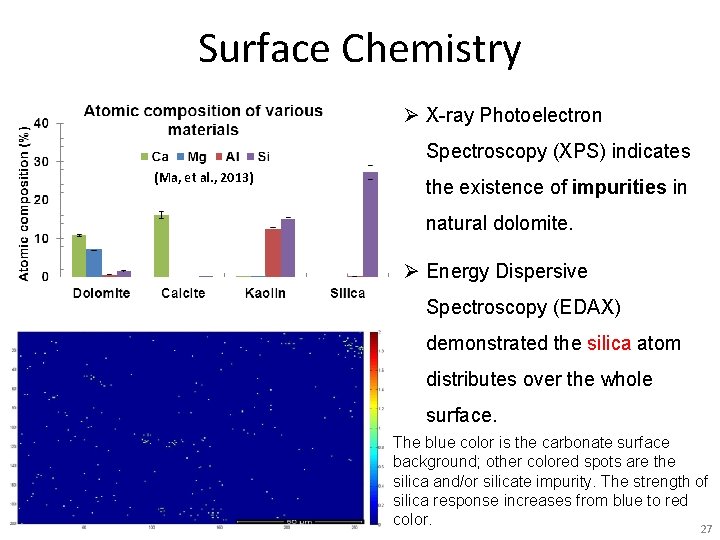
Surface Chemistry Ø X-ray Photoelectron Spectroscopy (XPS) indicates (Ma, et al. , 2013) the existence of impurities in natural dolomite. Ø Energy Dispersive Spectroscopy (EDAX) demonstrated the silica atom distributes over the whole surface. The blue color is the carbonate surface background; other colored spots are the silica and/or silicate impurity. The strength of silica response increases from blue to red SPE-169040 -MS, Adsorption of a Switchable Cationic Surfactant on Natural Carbonate Minerals, Leyu Cui color. 27
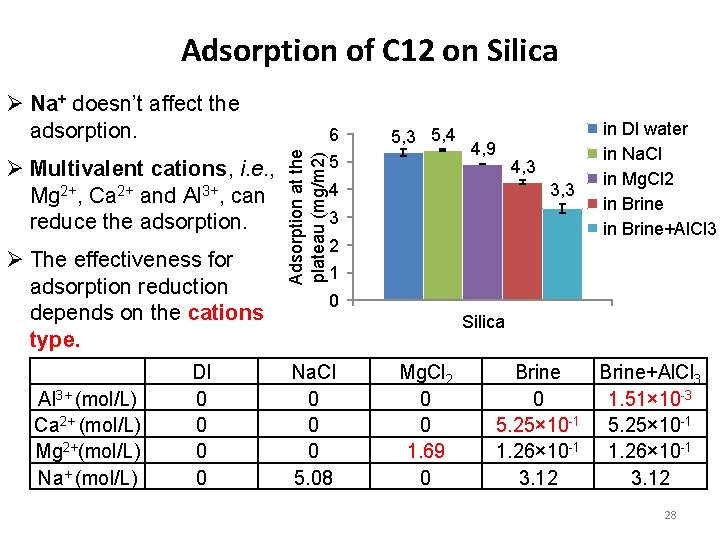
Adsorption of C 12 on Silica Ø Na+ doesn’t affect the adsorption. Ø The effectiveness for adsorption reduction depends on the cations type. Al 3+ (mol/L) Ca 2+ (mol/L) Mg 2+(mol/L) Na+ (mol/L) DI 0 0 Adsorption at the plateau (mg/m 2) Ø Multivalent cations, i. e. , Mg 2+, Ca 2+ and Al 3+, can reduce the adsorption. 6 5, 3 5, 4 5 4, 9 4, 3 3, 3 4 3 2 in DI water in Na. Cl in Mg. Cl 2 in Brine+Al. Cl 3 1 0 Silica Na. Cl 0 0 0 5. 08 Mg. Cl 2 0 0 1. 69 0 Brine+Al. Cl 3 0 1. 51× 10 -3 5. 25× 10 -1 1. 26× 10 -1 3. 12 28
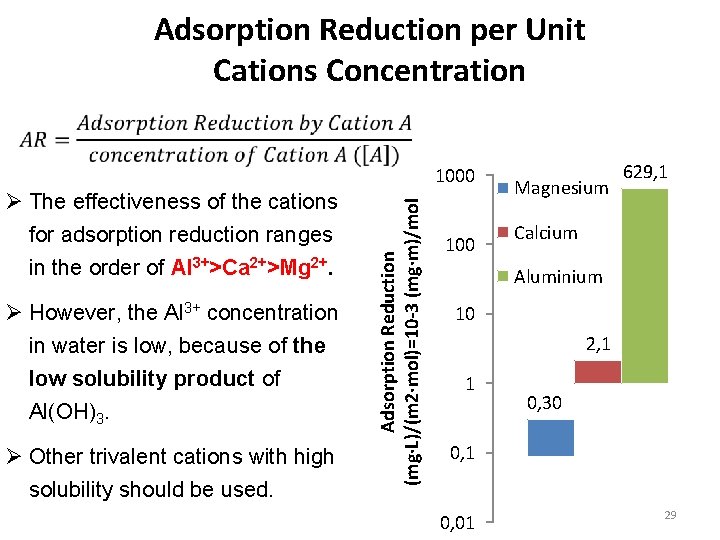
Adsorption Reduction per Unit Cations Concentration Ø The effectiveness of the cations for adsorption reduction ranges in the order of Al 3+>Ca 2+>Mg 2+. Ø However, the Al 3+ concentration in water is low, because of the low solubility product of Al(OH)3. Ø Other trivalent cations with high solubility should be used. Adsorption Reduction (mg·L)/(m 2·mol)=10 -3 (mg·m)/mol 1000 100 Magnesium 629, 1 Calcium Aluminium 10 2, 1 1 0, 30 0, 1 0, 01 29
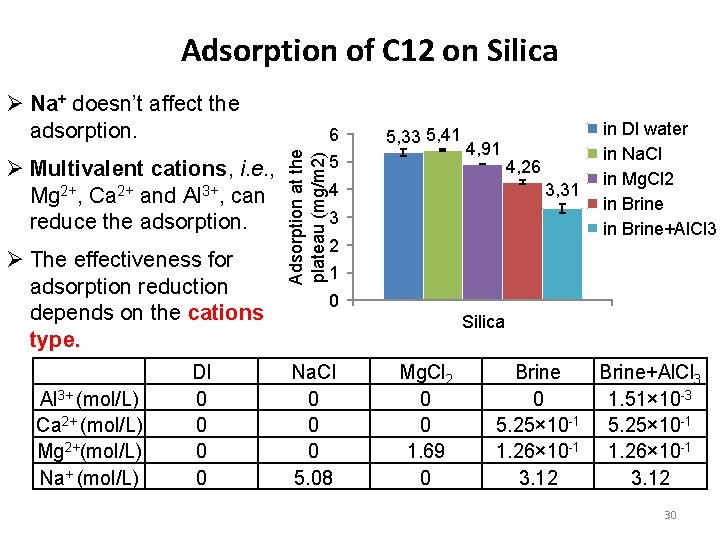
Adsorption of C 12 on Silica Ø Na+ doesn’t affect the adsorption. Ø The effectiveness for adsorption reduction depends on the cations type. Al 3+ (mol/L) Ca 2+ (mol/L) Mg 2+(mol/L) Na+ (mol/L) DI 0 0 Adsorption at the plateau (mg/m 2) Ø Multivalent cations, i. e. , Mg 2+, Ca 2+ and Al 3+, can reduce the adsorption. 6 5, 33 5, 41 5 4, 91 4, 26 3, 31 4 3 2 in DI water in Na. Cl in Mg. Cl 2 in Brine+Al. Cl 3 1 0 Silica Na. Cl 0 0 0 5. 08 Mg. Cl 2 0 0 1. 69 0 Brine+Al. Cl 3 0 1. 51× 10 -3 5. 25× 10 -1 1. 26× 10 -1 3. 12 30
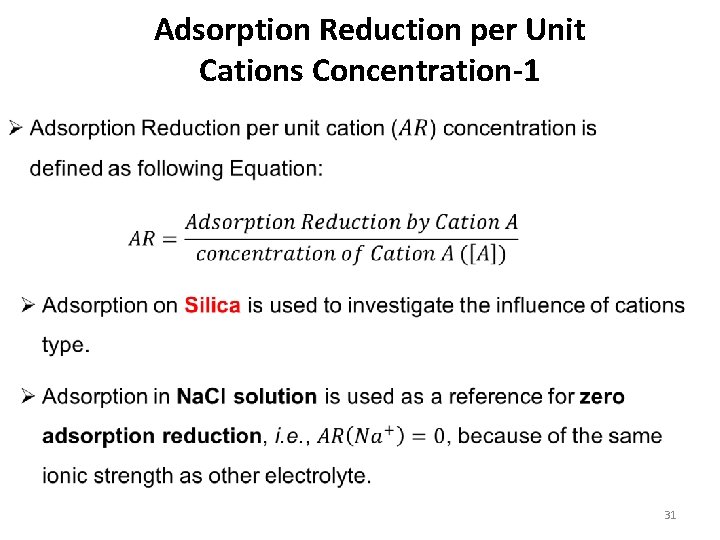
Adsorption Reduction per Unit Cations Concentration-1 31
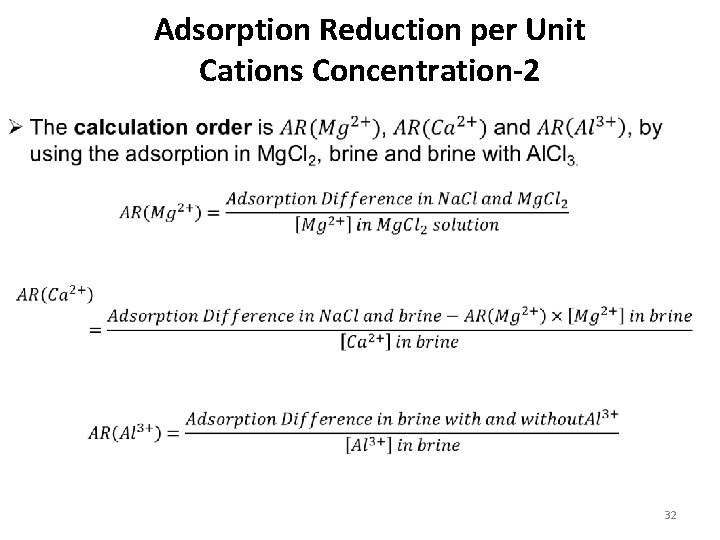
Adsorption Reduction per Unit Cations Concentration-2 32
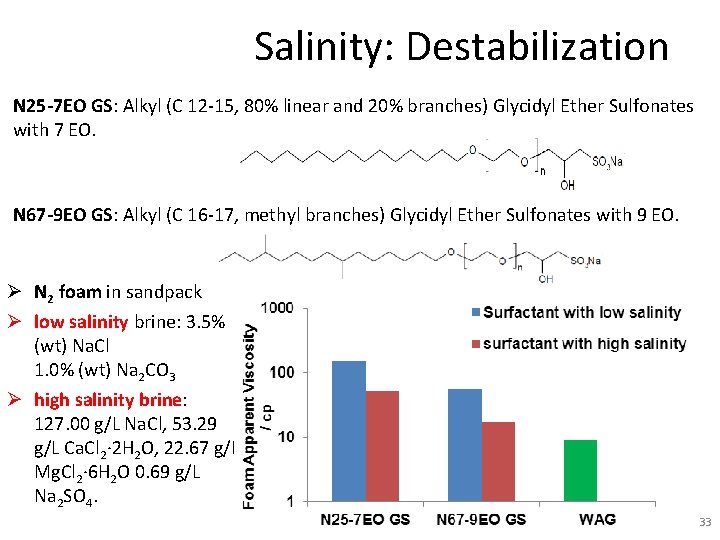
Salinity: Destabilization N 25 -7 EO GS: Alkyl (C 12 -15, 80% linear and 20% branches) Glycidyl Ether Sulfonates with 7 EO. N 67 -9 EO GS: Alkyl (C 16 -17, methyl branches) Glycidyl Ether Sulfonates with 9 EO. Ø N 2 foam in sandpack Ø low salinity brine: 3. 5% (wt) Na. Cl 1. 0% (wt) Na 2 CO 3 Ø high salinity brine: 127. 00 g/L Na. Cl, 53. 29 g/L Ca. Cl 2∙ 2 H 2 O, 22. 67 g/L Mg. Cl 2∙ 6 H 2 O 0. 69 g/L Na 2 SO 4. 33

Mineral Dissolution Ø The carbonate mineral was dissolved in DI water and CO 2. Ø The sufficient divalent cations, i. e. , Ca 2+ and Mg 2+, are suggested to be added in brine. 34
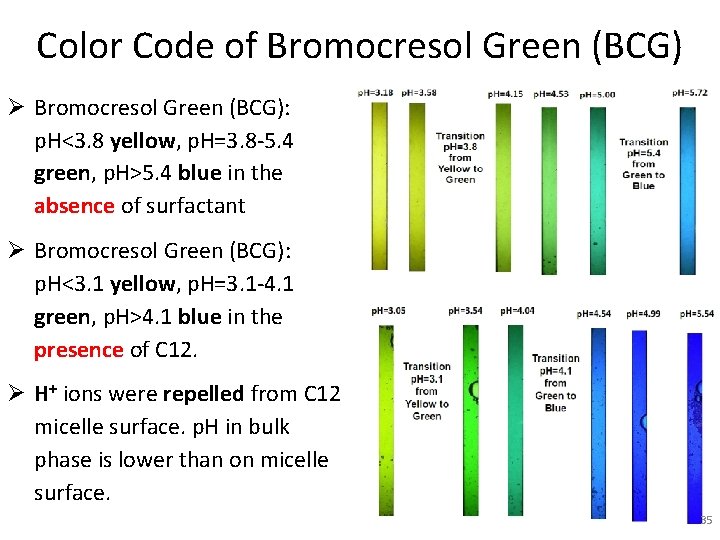
Color Code of Bromocresol Green (BCG) Ø Bromocresol Green (BCG): p. H<3. 8 yellow, p. H=3. 8 -5. 4 green, p. H>5. 4 blue in the absence of surfactant Ø Bromocresol Green (BCG): p. H<3. 1 yellow, p. H=3. 1 -4. 1 green, p. H>4. 1 blue in the presence of C 12. Ø H+ ions were repelled from C 12 micelle surface. p. H in bulk phase is lower than on micelle surface. 35
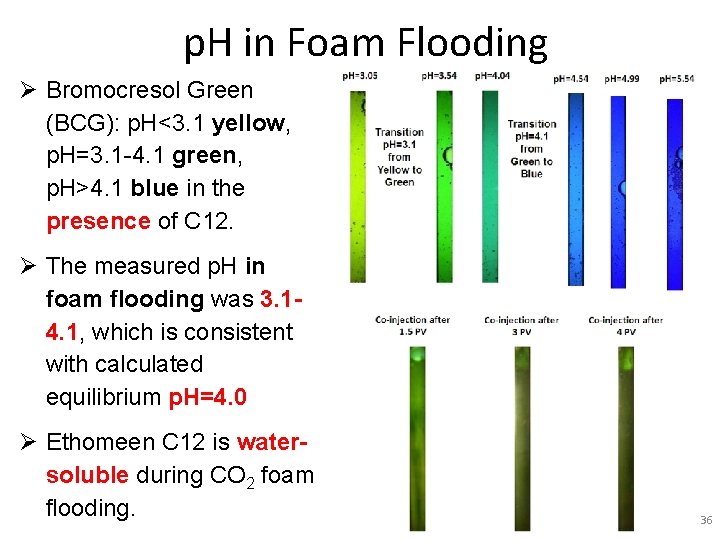
p. H in Foam Flooding Ø Bromocresol Green (BCG): p. H<3. 1 yellow, p. H=3. 1 -4. 1 green, p. H>4. 1 blue in the presence of C 12. Ø The measured p. H in foam flooding was 3. 14. 1, which is consistent with calculated equilibrium p. H=4. 0 Ø Ethomeen C 12 is watersoluble during CO 2 foam flooding. 36
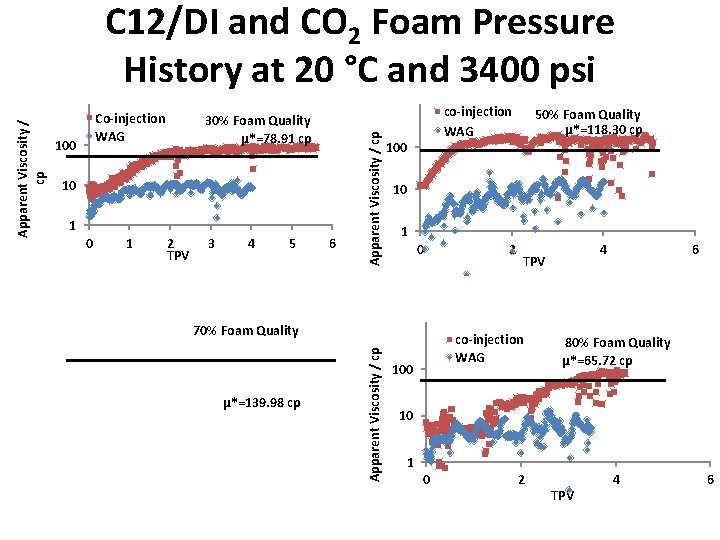
Co-injection WAG 100 30% Foam Quality 10 1 2 TPV 3 4 5 6 Apparent Viscosity / cp C 12/DI and CO 2 Foam Pressure History at 20 °C and 3400 psi TPV
- Slides: 37