CO 2 Capture from Flue Gas using Amino





![Validating Equipment at 40 ºC [1]. Leila Faramarzi, Georgios M. Kontogeorgis, Kaj Thomsen, Erling Validating Equipment at 40 ºC [1]. Leila Faramarzi, Georgios M. Kontogeorgis, Kaj Thomsen, Erling](https://slidetodoc.com/presentation_image/16df61417d5dc710fff1fb5002735d19/image-15.jpg)



- Slides: 27

CO 2 Capture from Flue Gas using Amino Acid Salt Solutions Benedicte Mai Lerche Kaj Thomsen & Erling H. Stenby

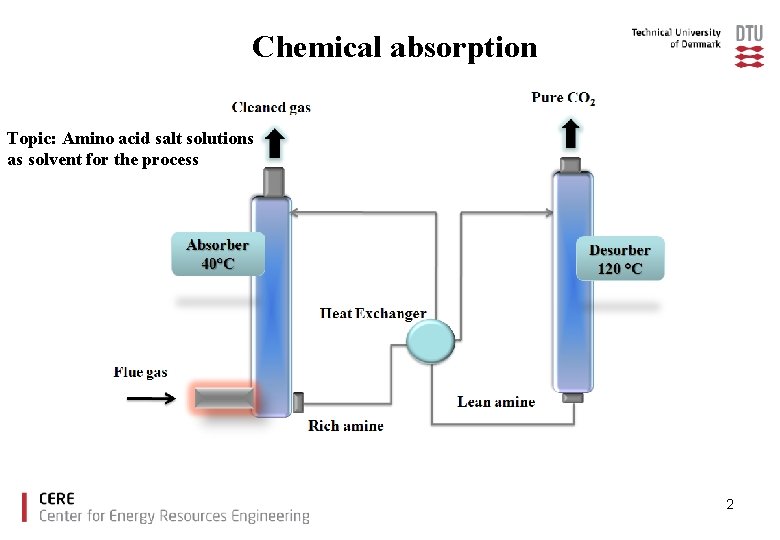
Chemical absorption Topic: Amino acid salt solutions as solvent for the process 2

Solvent properties Ø Volatility Ø Stability Ø CO 2 absorption rate Ø CO 2 solubility Ø CO 2 desorption ability CO 2 loading (mol CO 2/mol amine) CO 2 capacity (mol CO 2/Kg solution) 3

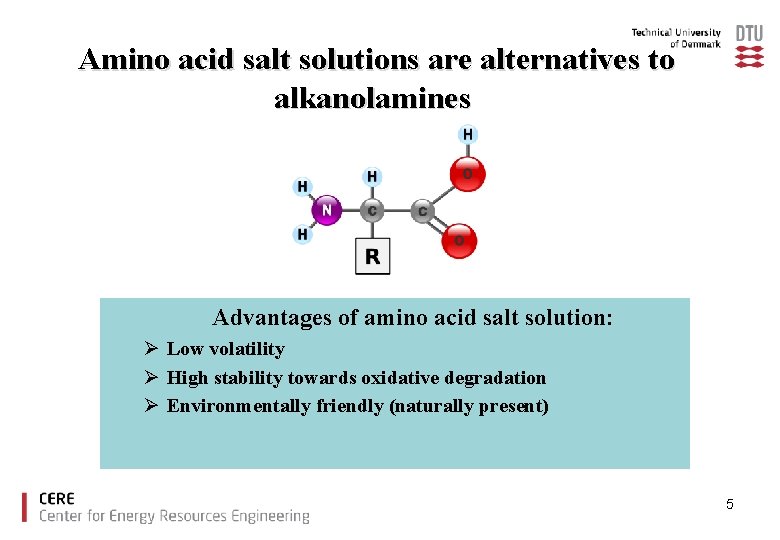
Available solvents are almost exclusively based on alkanolamines Mono-ethanolamine MEA The alkanolamine most widely used for CO 2 capture Disadvantages of alkanolamines: Ø Volatile (Loss of solvent during the regeneration process) Ø Degraded by the oxygen (Loss of solvent) Ø Toxic degradation products (Create environmental concern) 4

Amino acid salt solutions are alternatives to alkanolamines Advantages of amino acid salt solution: Ø Low volatility Ø High stability towards oxidative degradation Ø Environmentally friendly (naturally present) 5

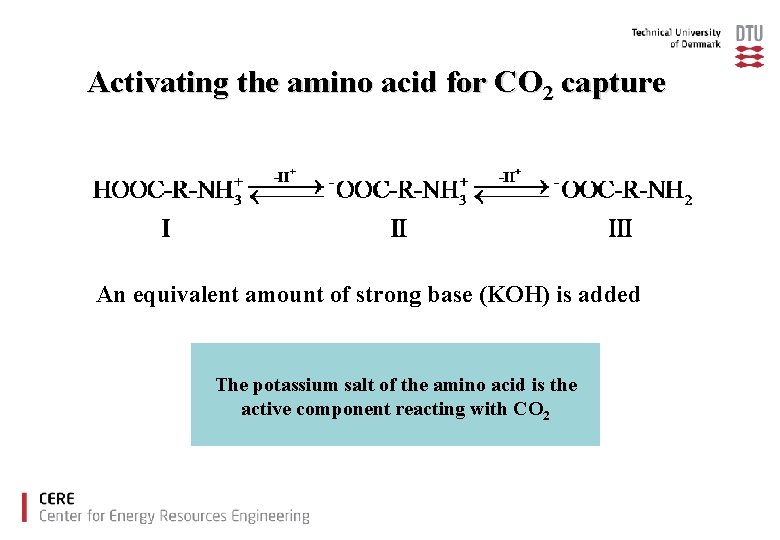
Activating the amino acid for CO 2 capture An equivalent amount of strong base (KOH) is added The potassium salt of the amino acid is the active component reacting with CO 2

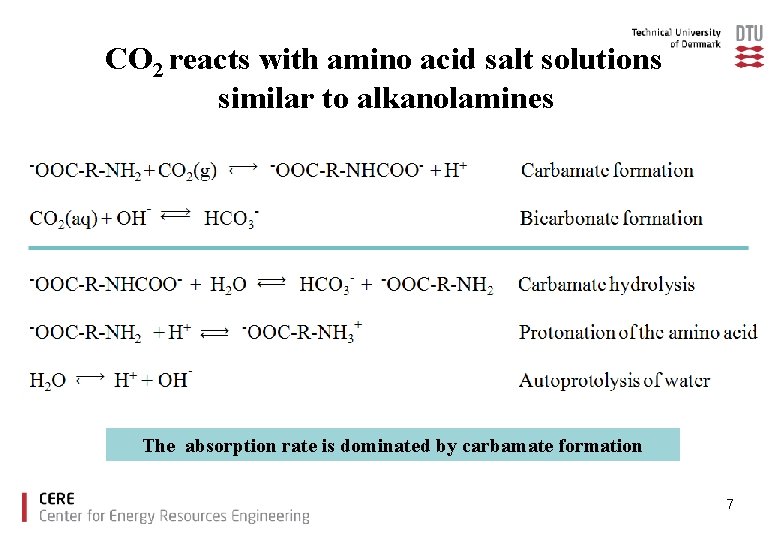
CO 2 reacts with amino acid salt solutions similar to alkanolamines The absorption rate is dominated by carbamate formation 7

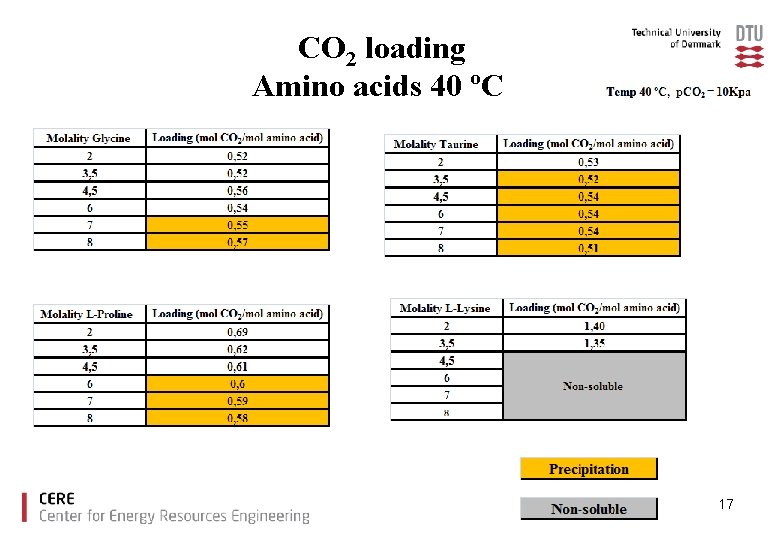
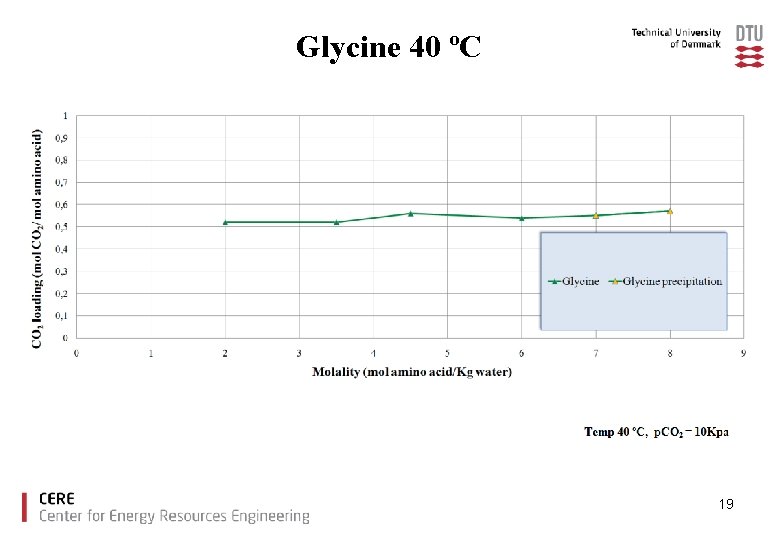
Precipitation of the reaction products can occur with high amino acid salt concentration at high CO 2 loading Precipitation offers certain interesting opportunities as well as drawbacks: Opportunities: Ø Increase of CO 2 loading capacity Drawbacks: Ø plugging of the equipment. 8

Selecting amino acid salt solutions for CO 2 capture CO 2 loading Cyclic absorption & regeneration Heat stability Solubility 9

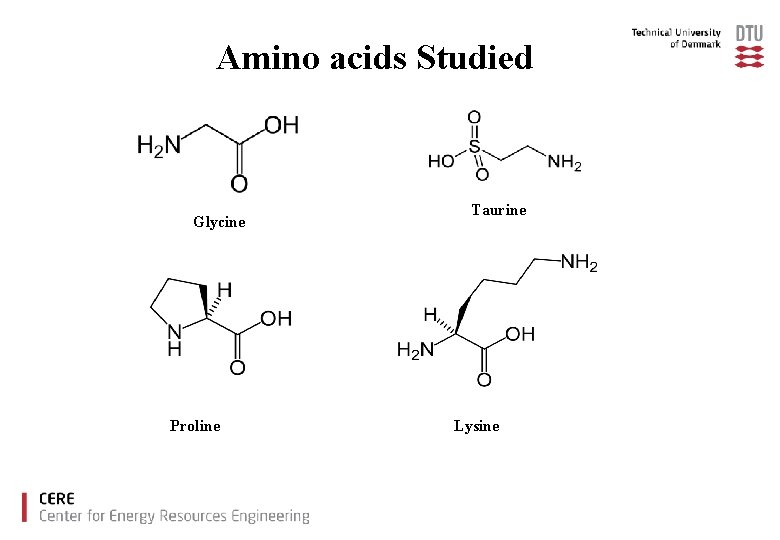
Amino acids Studied Glycine Proline Taurine Lysine

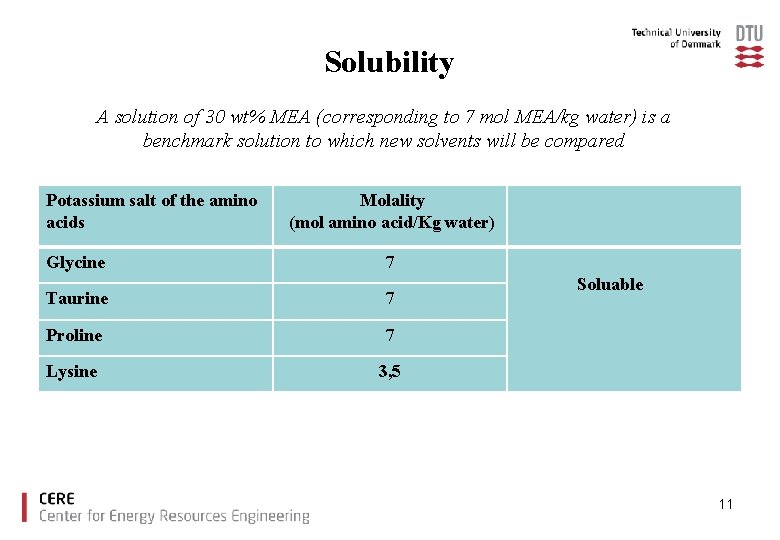
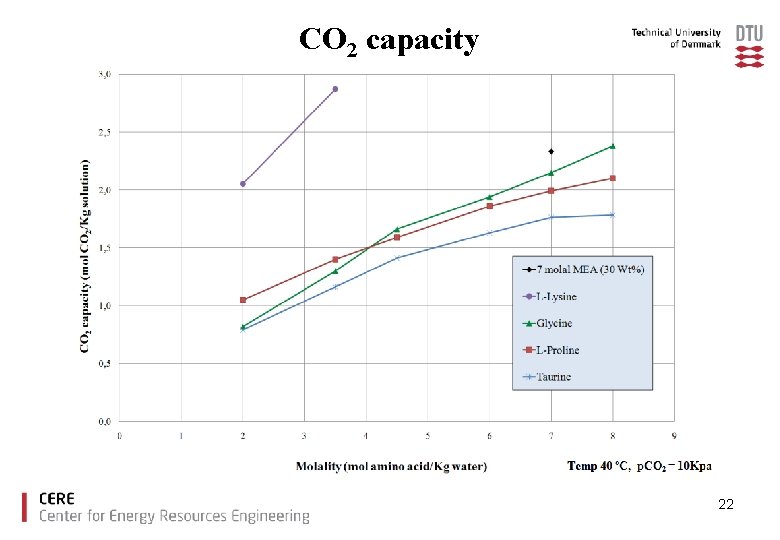
Solubility A solution of 30 wt% MEA (corresponding to 7 mol MEA/kg water) is a benchmark solution to which new solvents will be compared Potassium salt of the amino acids Glycine Molality (mol amino acid/Kg water) 7 Taurine 7 Proline 7 Lysine 3, 5 Soluable 11

Heat stability (amino acid analysis) Binds to the amine group OPA flourocense reagent pump Buffer A Oven (62ºC) pump p. H gradient: 3. 1 - 10. 2 sample holder ion exchange column Negative charged coil Buffer B pump Separation based on iso-electric point Florescence detector Data treatment waste 12

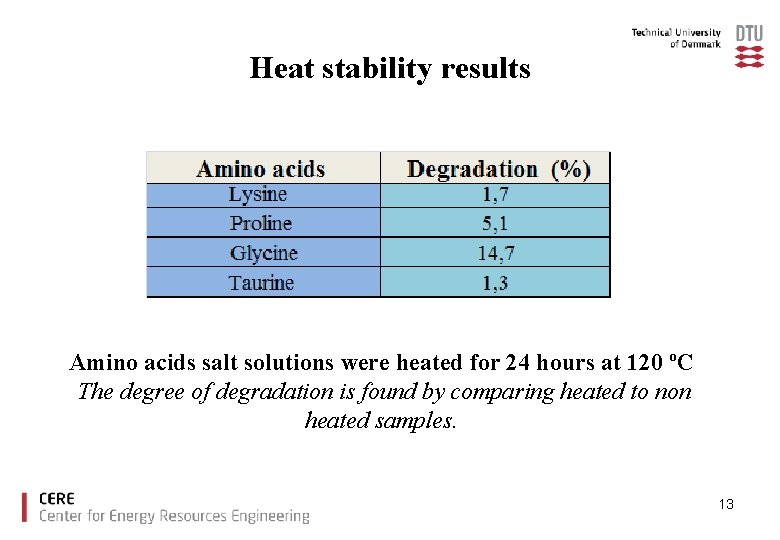
Heat stability results Amino acids salt solutions were heated for 24 hours at 120 ºC The degree of degradation is found by comparing heated to non heated samples. 13

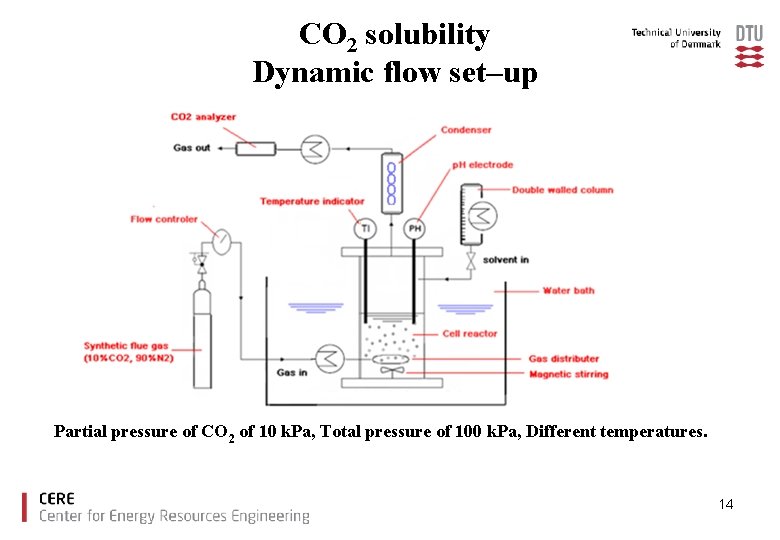
CO 2 solubility Dynamic flow set–up Partial pressure of CO 2 of 10 k. Pa, Total pressure of 100 k. Pa, Different temperatures. 14
![Validating Equipment at 40 ºC 1 Leila Faramarzi Georgios M Kontogeorgis Kaj Thomsen Erling Validating Equipment at 40 ºC [1]. Leila Faramarzi, Georgios M. Kontogeorgis, Kaj Thomsen, Erling](https://slidetodoc.com/presentation_image/16df61417d5dc710fff1fb5002735d19/image-15.jpg)
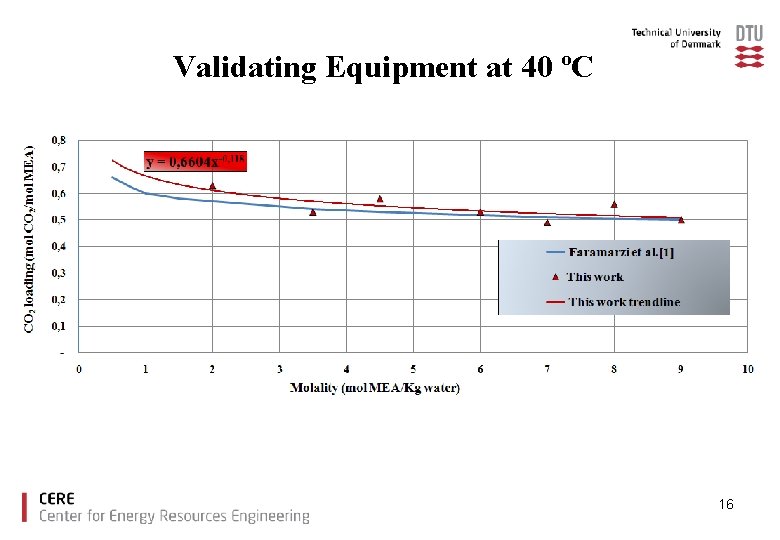
Validating Equipment at 40 ºC [1]. Leila Faramarzi, Georgios M. Kontogeorgis, Kaj Thomsen, Erling H. Stenby, “Extended UNIQUAC model for thermodynamic modeling of CO 2 absorption in aqueous alkanolamine solutions” Fluid Phase Equilibria, 2009, vol. 282 pp. 121– 132 15

Validating Equipment at 40 ºC 16

CO 2 loading Amino acids 40 ºC 17

CO 2 loading 40 ºC 18

Glycine 40 ºC 19

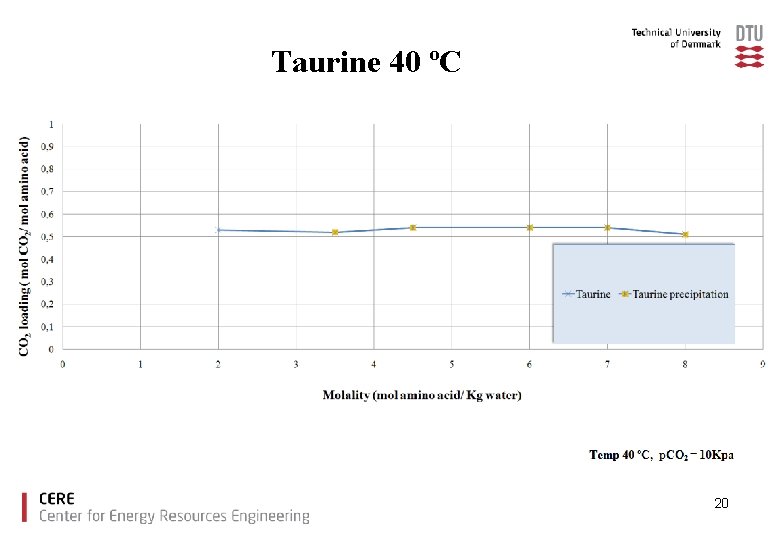
Taurine 40 ºC 20

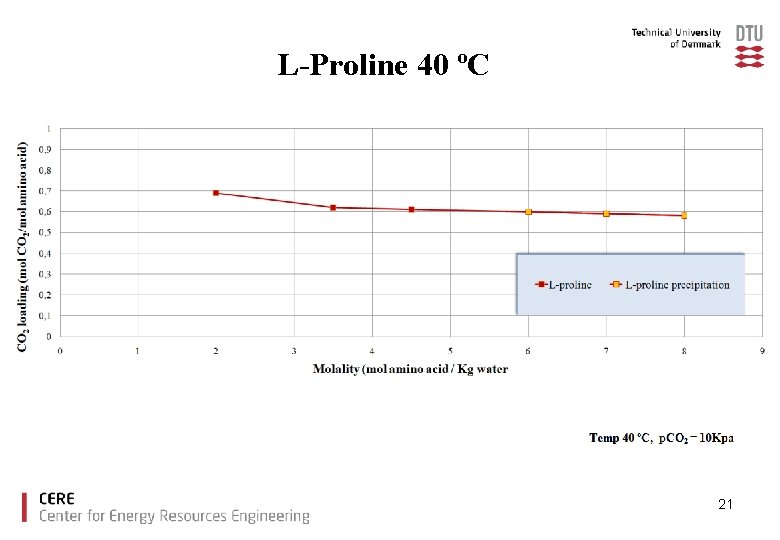
L-Proline 40 ºC 21

CO 2 capacity 22

Conclusions Ø The amino acids tested showed good CO 2 loading capacities compared to MEA. Ø With increased amino acid salt concentration precipitation was observed for glycine, taurine and proline. Ø There is no increase in CO 2 loading capacity due to precipitation under the experimental conditions used. Ø Lysine offers high CO 2 capacity without precipitation. 23

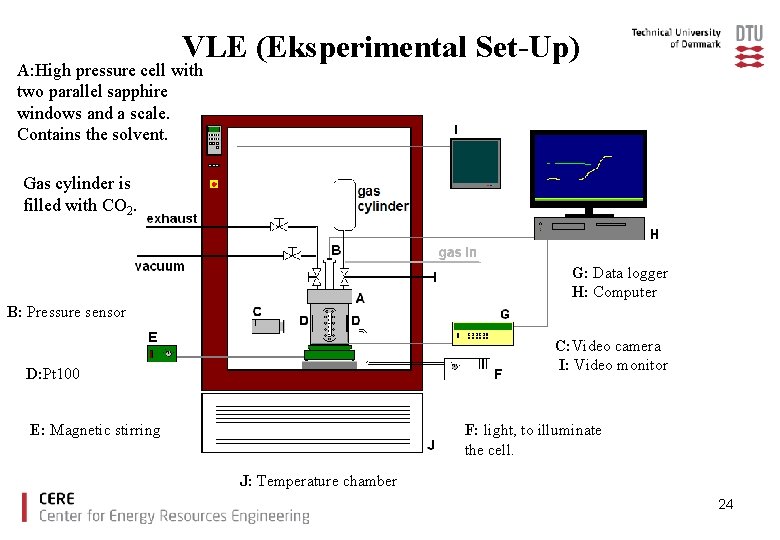
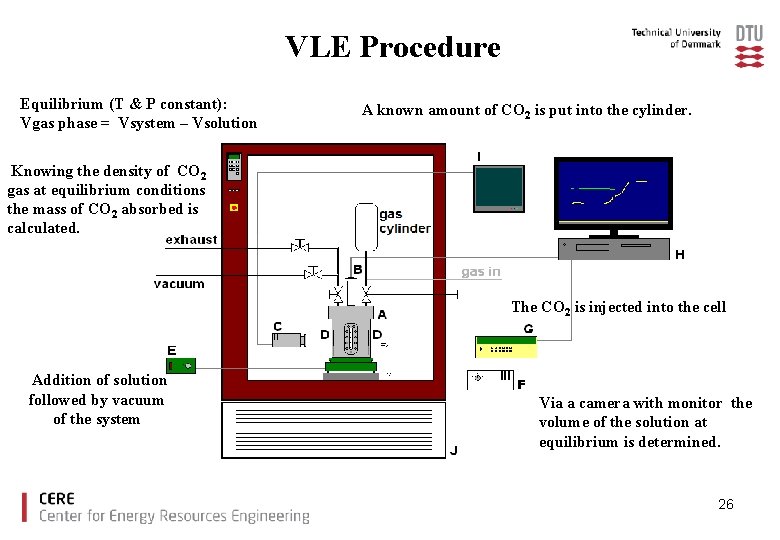
VLE (Eksperimental Set-Up) A: High pressure cell with two parallel sapphire windows and a scale. Contains the solvent. Gas cylinder is filled with CO 2. G: Data logger H: Computer B: Pressure sensor C: Video camera I: Video monitor D: Pt 100 E: Magnetic stirring F: light, to illuminate the cell. J: Temperature chamber 24

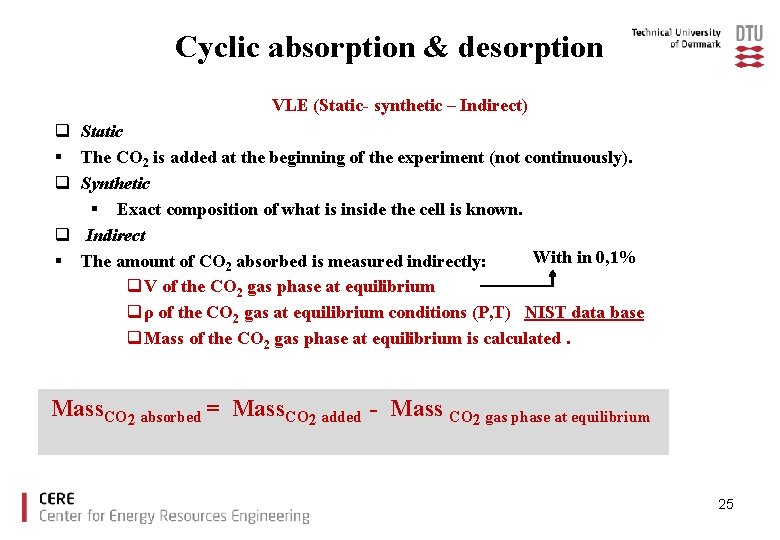
Cyclic absorption & desorption VLE (Static- synthetic – Indirect) q Static § The CO 2 is added at the beginning of the experiment (not continuously). q Synthetic § Exact composition of what is inside the cell is known. q Indirect With in 0, 1% § The amount of CO 2 absorbed is measured indirectly: q V of the CO 2 gas phase at equilibrium q ρ of the CO 2 gas at equilibrium conditions (P, T) NIST data base q Mass of the CO 2 gas phase at equilibrium is calculated. Mass. CO 2 absorbed = Mass. CO 2 added - Mass CO 2 gas phase at equilibrium 25

VLE Procedure Equilibrium (T & P constant): Vgas phase = Vsystem – Vsolution A known amount of CO 2 is put into the cylinder. Knowing the density of CO 2 gas at equilibrium conditions the mass of CO 2 absorbed is calculated. The CO 2 is injected into the cell Addition of solution followed by vacuum of the system Via a camera with monitor the volume of the solution at equilibrium is determined. 26

Thank you for your attention! 27