Cluster Phases Gels and Yukawa Glasses in charged



















- Slides: 60

Cluster Phases, Gels and Yukawa Glasses in charged colloid-polymer mixtures. Francesco Sciortino

Motivations Dynamic Arrest in Colloidal Systems: Glasses and Gels Excluded Volume Short Range Attraction (SRA) SRA+ Longer Range Repulsion Investigate the competing effects of short range attraction and longer-range repulsion in colloidal systems Dynamics close to arrested states of matter: Cluster Phases, Glasses and/or Gels

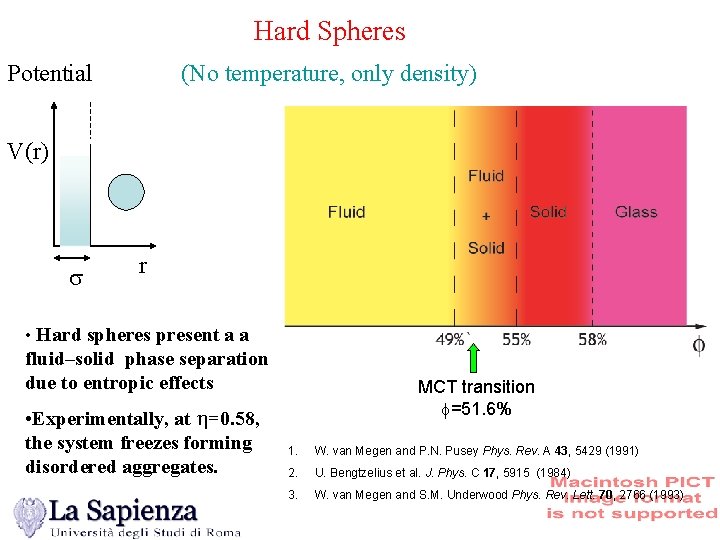
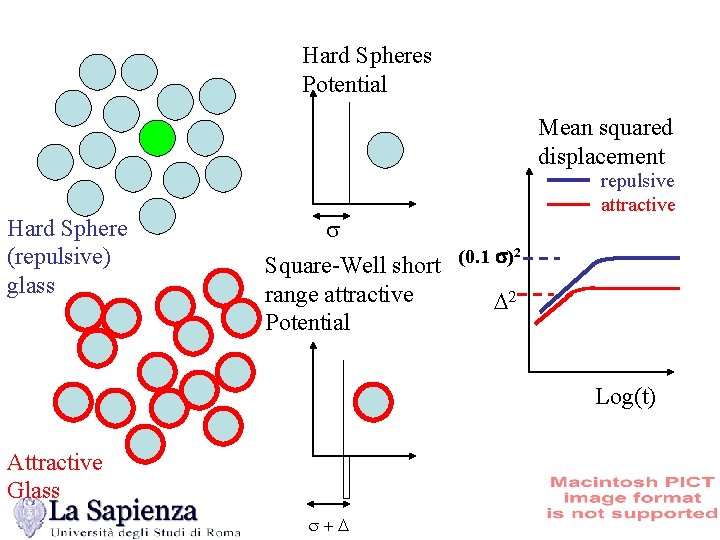
Hard Spheres Potential (No temperature, only density) V(r) s r • Hard spheres present a a fluid–solid phase separation due to entropic effects • Experimentally, at h=0. 58, the system freezes forming disordered aggregates. MCT transition =51. 6% 1. W. van Megen and P. N. Pusey Phys. Rev. A 43, 5429 (1991) 2. U. Bengtzelius et al. J. Phys. C 17, 5915 (1984) 3. W. van Megen and S. M. Underwood Phys. Rev. Lett. 70, 2766 (1993)

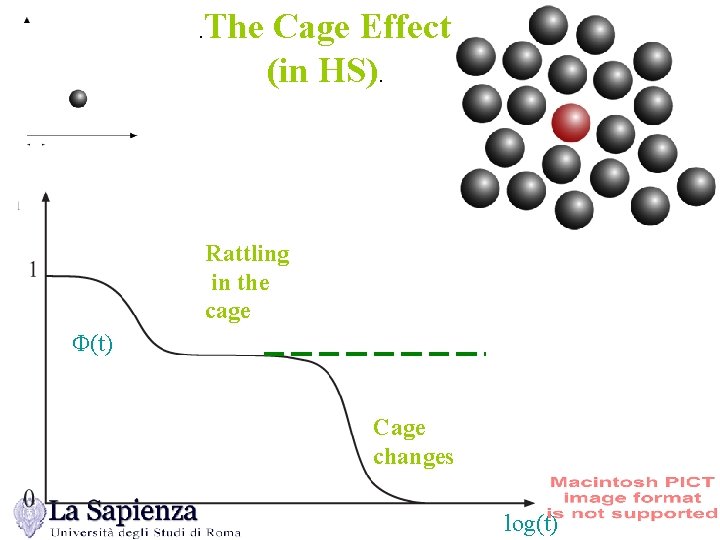
The Cage Effect (in HS). . Rattling in the cage F(t) Cage changes log(t)

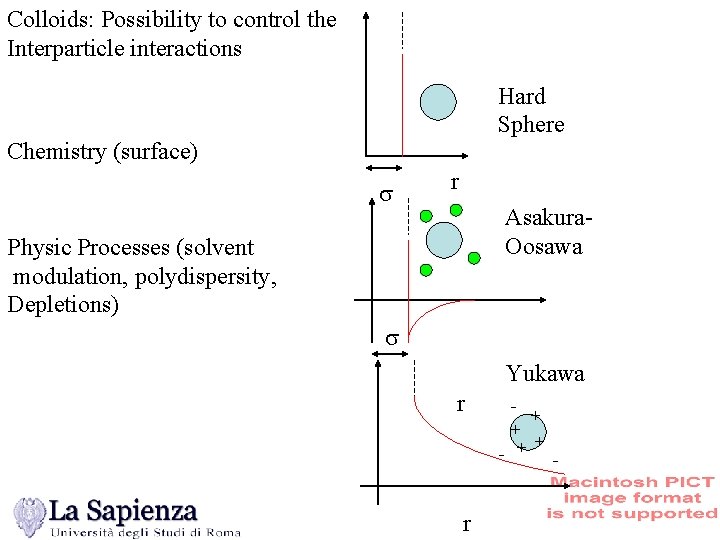
Colloids: Possibility to control the Interparticle interactions Hard Sphere Chemistry (surface) s r Asakura. Oosawa Physic Processes (solvent modulation, polydispersity, Depletions) s Yukawa r + + - + + r

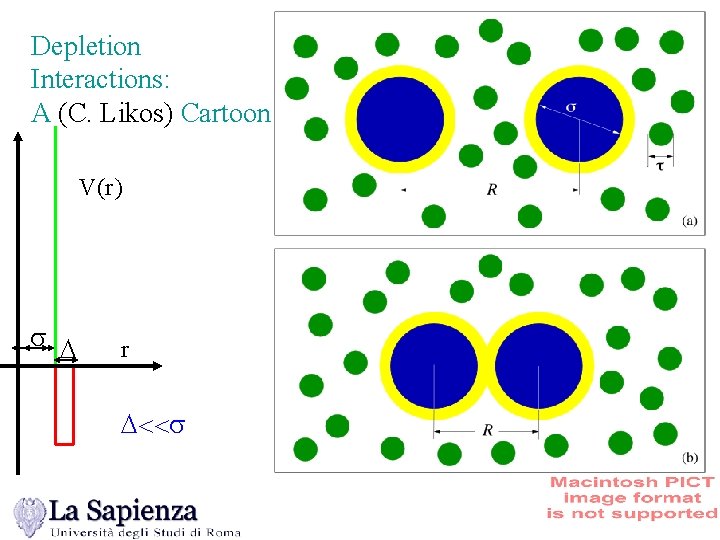
Depletion Interactions: A (C. Likos) Cartoon V(r ) s D r D<<s

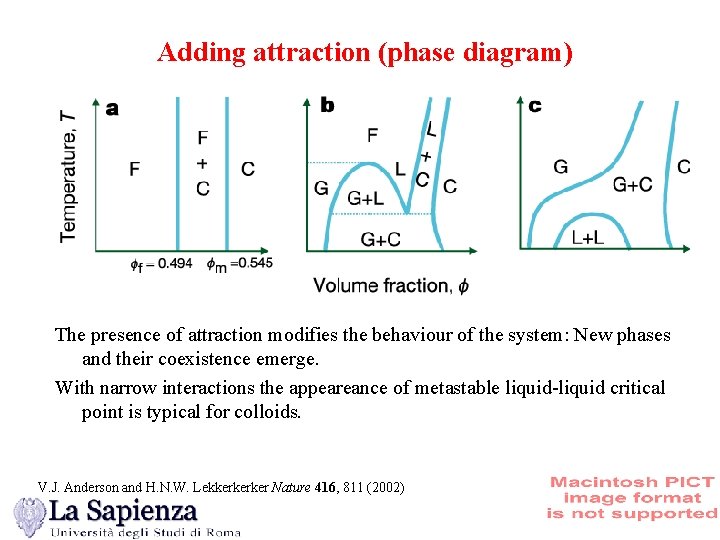
Adding attraction (phase diagram) The presence of attraction modifies the behaviour of the system: New phases and their coexistence emerge. With narrow interactions the appeareance of metastable liquid-liquid critical point is typical for colloids. V. J. Anderson and H. N. W. Lekkerkerker Nature 416, 811 (2002)


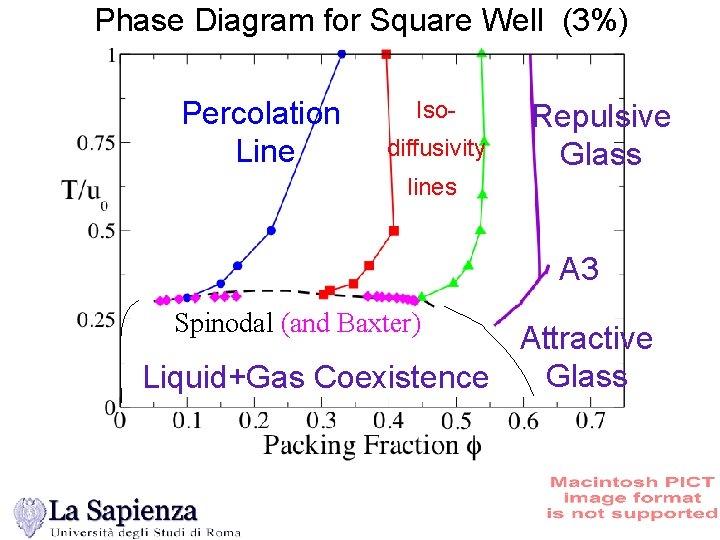
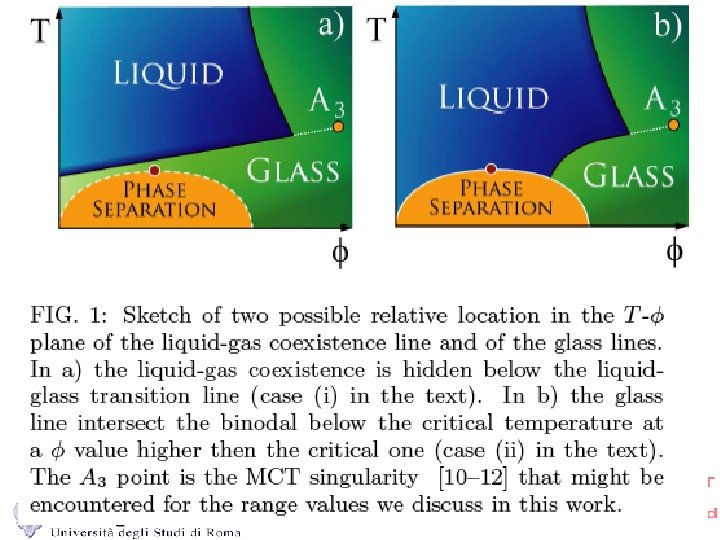
Phase Diagram for Square Well (3%) Percolation Line Isodiffusivity Repulsive Glass lines A 3 Spinodal (and Baxter) Attractive Glass Liquid+Gas Coexistence

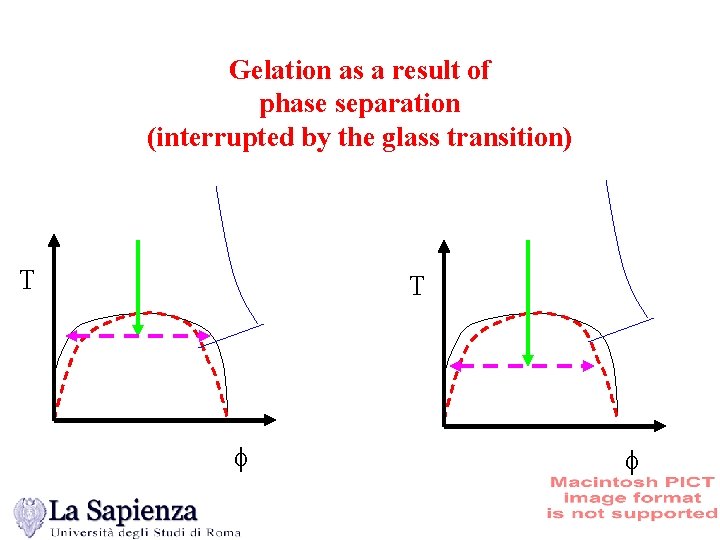
Gelation as a result of phase separation (interrupted by the glass transition) T T


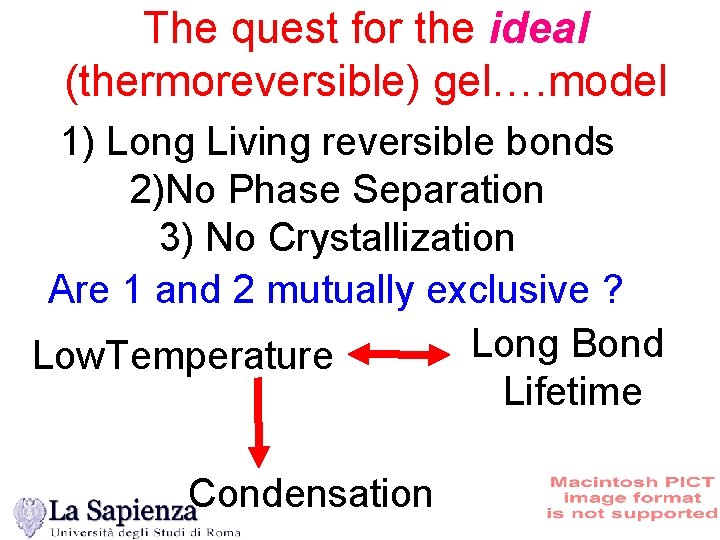
The quest for the ideal (thermoreversible) gel…. model 1) Long Living reversible bonds 2)No Phase Separation 3) No Crystallization Are 1 and 2 mutually exclusive ? Long Bond Low. Temperature Lifetime Condensation

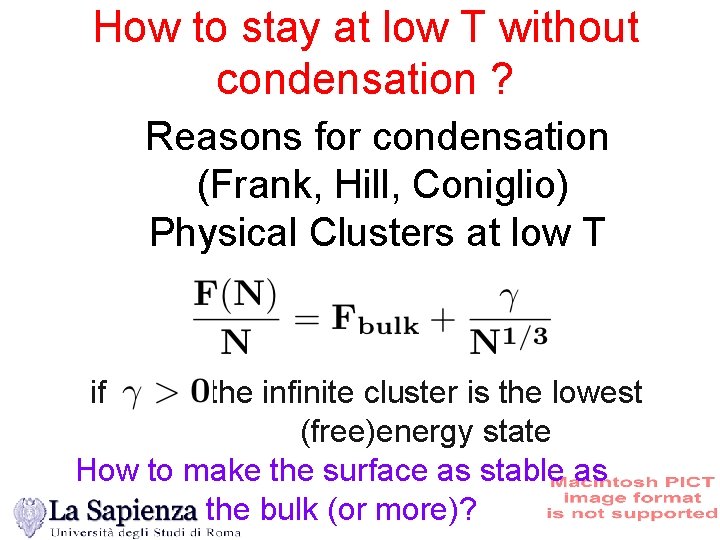
How to stay at low T without condensation ? Reasons for condensation (Frank, Hill, Coniglio) Physical Clusters at low T if the infinite cluster is the lowest (free)energy state How to make the surface as stable as the bulk (or more)?

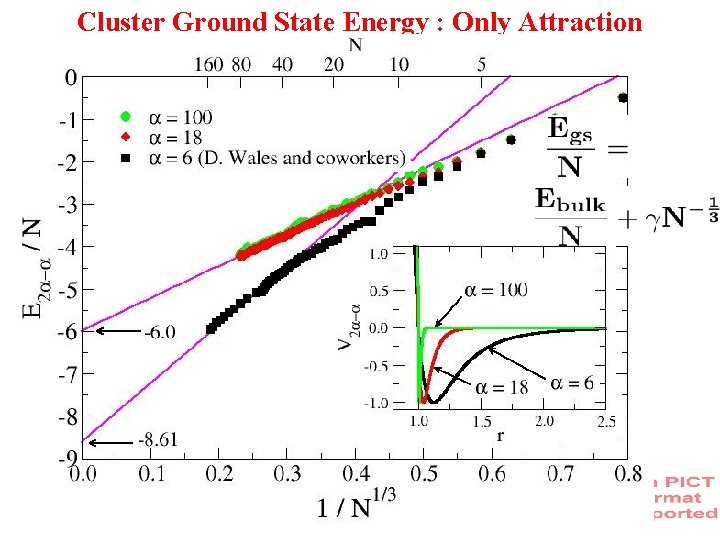
Cluster Ground State Energy : Only Attraction

Routes to Arrest at low packing fractions (in the absence of a “liquid-gas” phase separation) Competition between short range attraction and long-range repulsion (this talk) Limited Valency (see E. Zaccarelli et al PRL xxx

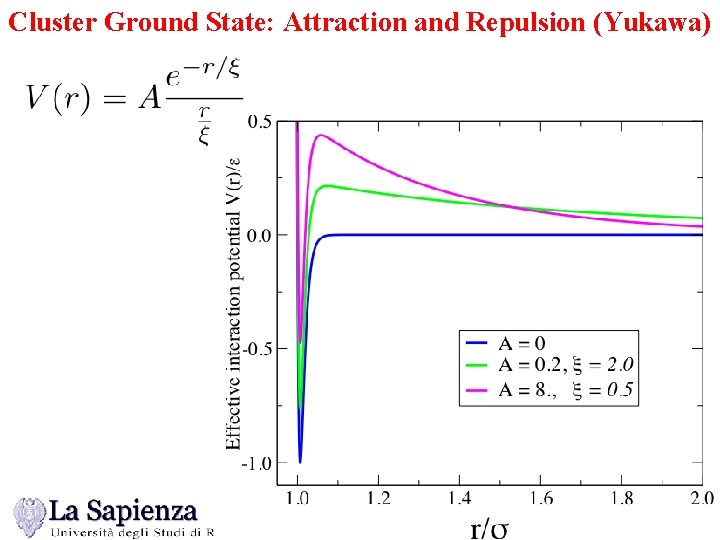
Cluster Ground State: Attraction and Repulsion (Yukawa)

Cluster Ground State: Attraction and Repulsion (Yukawa) Vanishing of the “surface tension” !

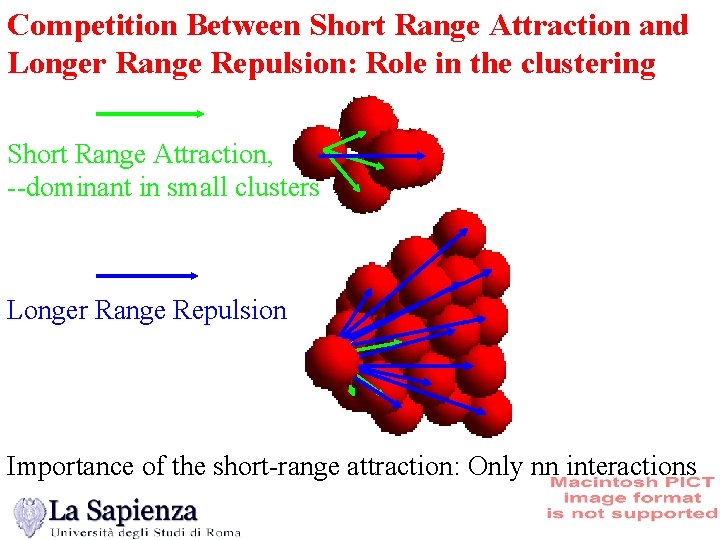
Competition Between Short Range Attraction and Longer Range Repulsion: Role in the clustering Short Range Attraction, --dominant in small clusters Longer Range Repulsion Importance of the short-range attraction: Only nn interactions

Typical Shapes in the ground state A=8 x =0. 5 s A=0. 05 x=2 s

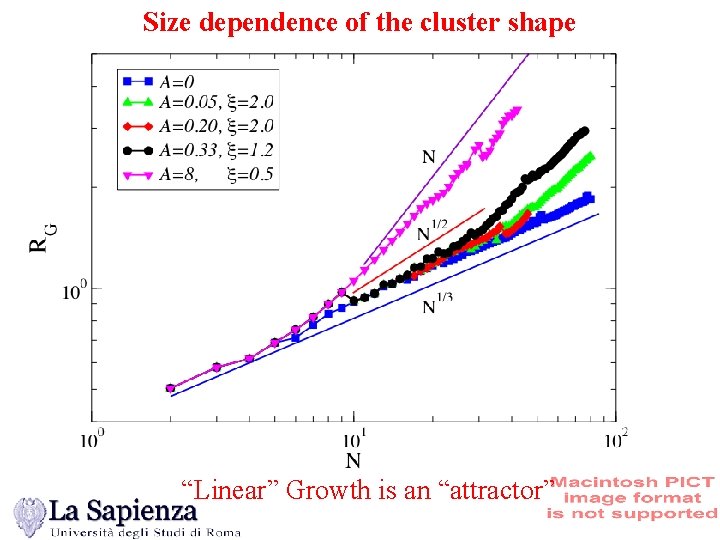
Size dependence of the cluster shape “Linear” Growth is an “attractor”

From isolated to interacting clusters Role of T and : On cooling (or on increasing attraction), monomers tend to cluster…. In the region of the phase diagram where the attractive potential would generate a phase separation…. repulsion slows down (or stop) aggregation. The range of the attractive interactions plays a role. How do clusters interact ?

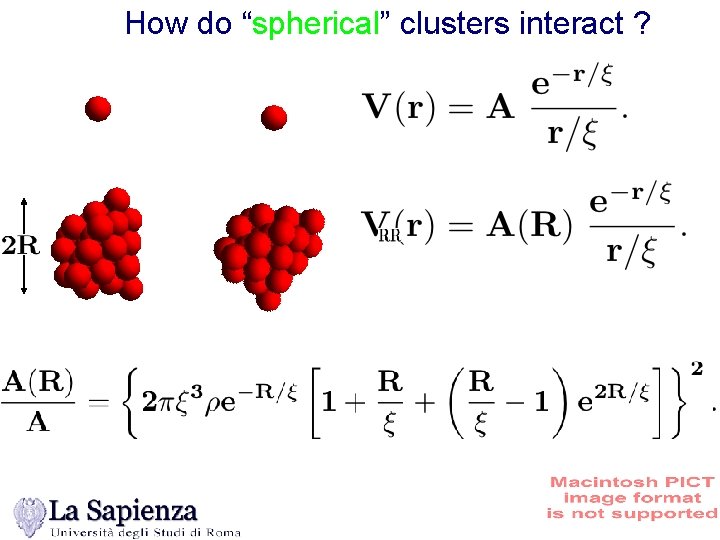
How do “spherical” clusters interact ?

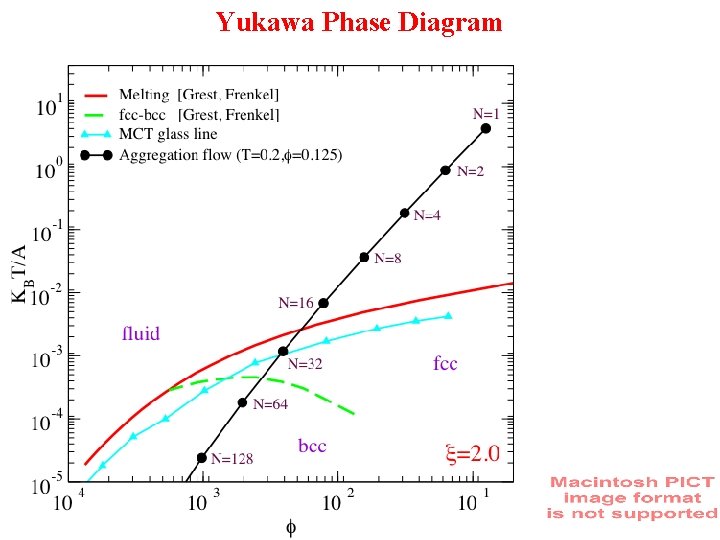
Yukawa Phase Diagram

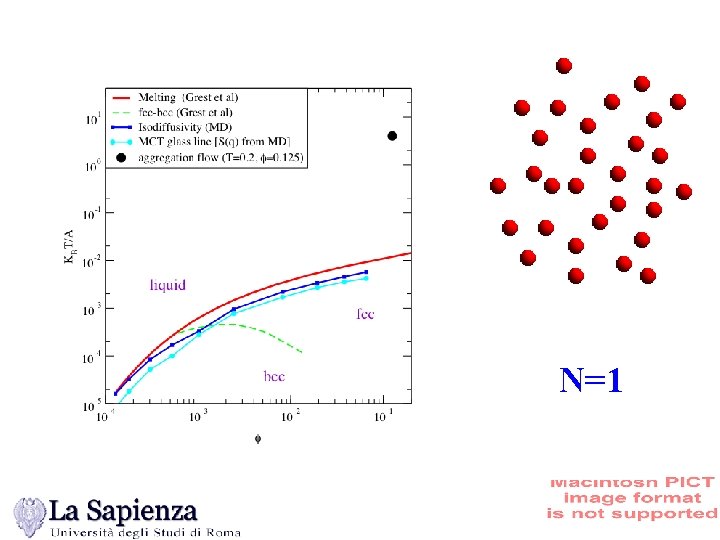
N=1

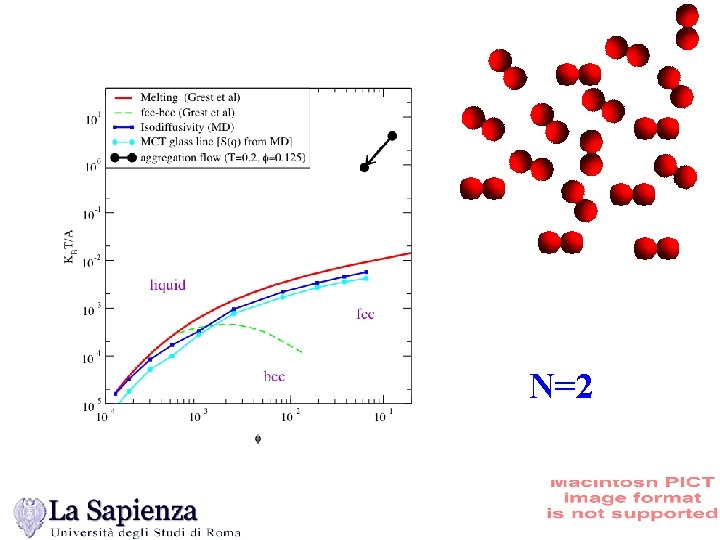
N=2

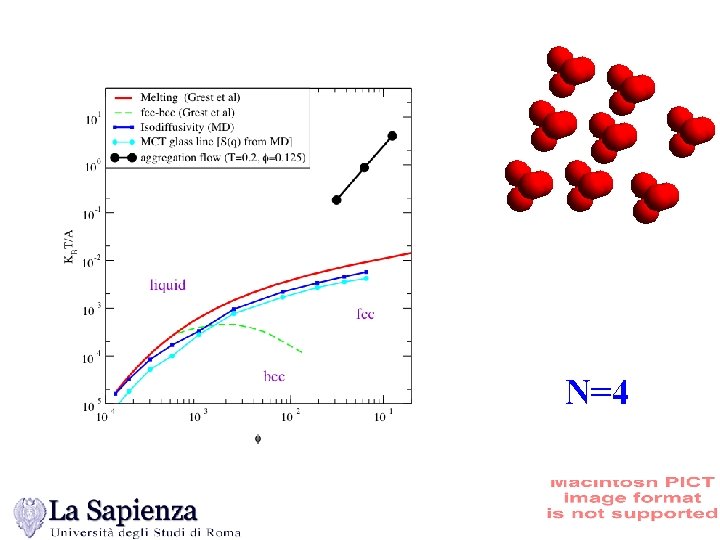
N=4

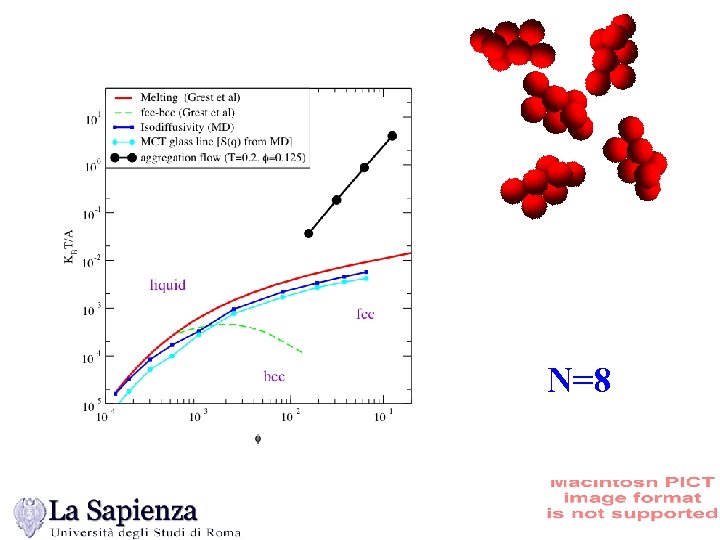
N=8

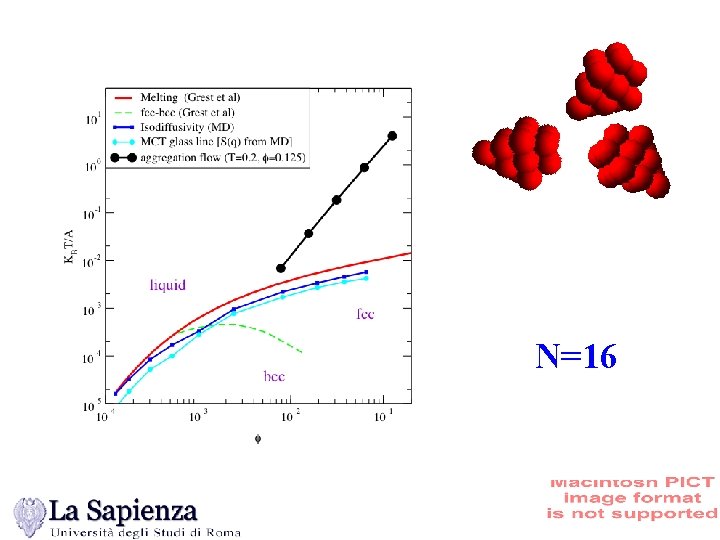
N=16

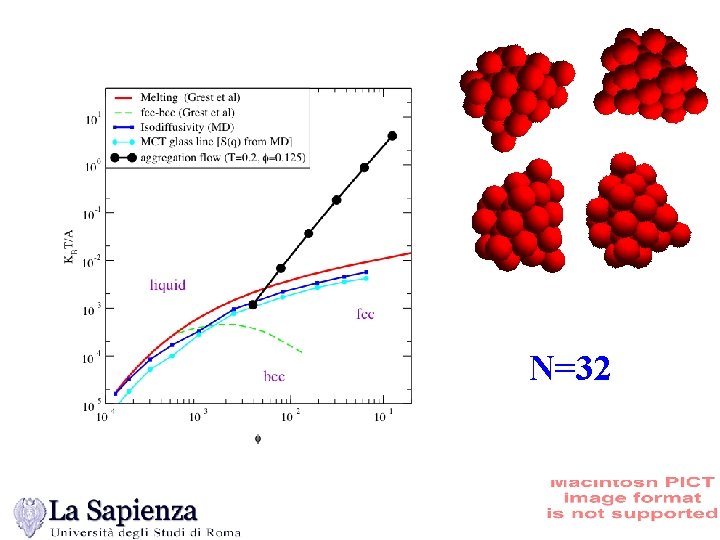
N=32

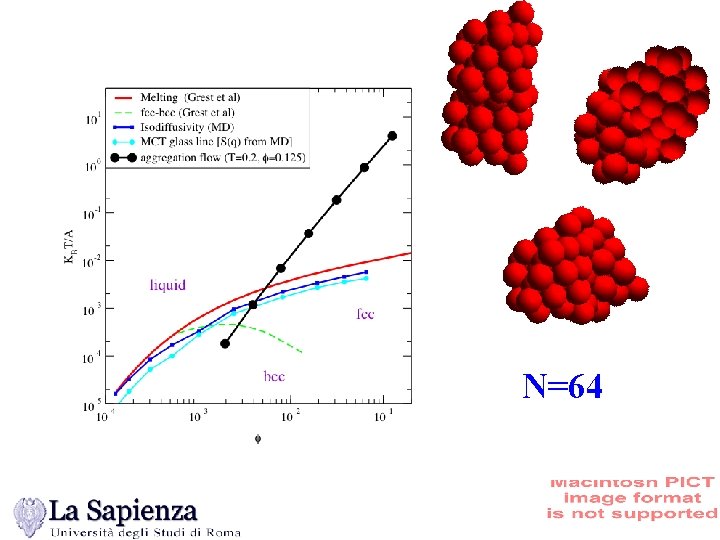
N=64

Yukawa Phase Diagram

lowering T Increasing packing fraction

Brief Intermediate Summary Equilibrium Cluster-phases result from the competition between aggregation and repulsion. Arrest at low packing fraction generated by a glass transition of the clusters. Aggregation progressively cool the system down till the repulsive cages become dominant

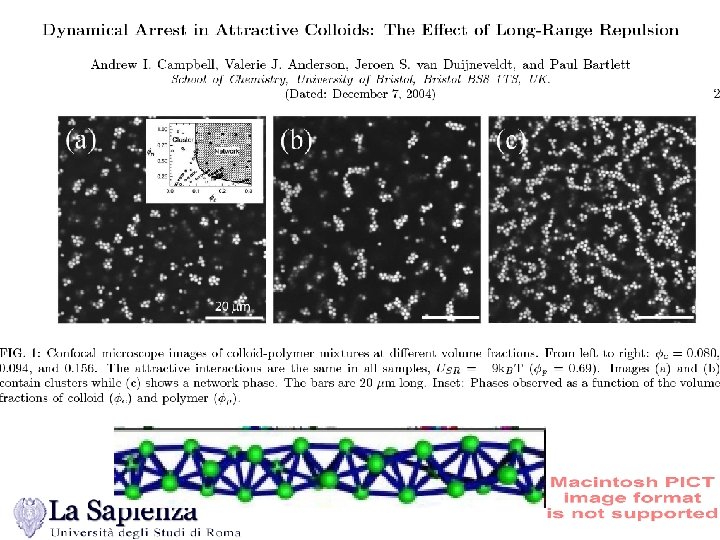
Interacting Clusters - Linear case The Bernal Spiral Campbell, Anderson, van Dujneveldt, Bartlett PRL June (2005)

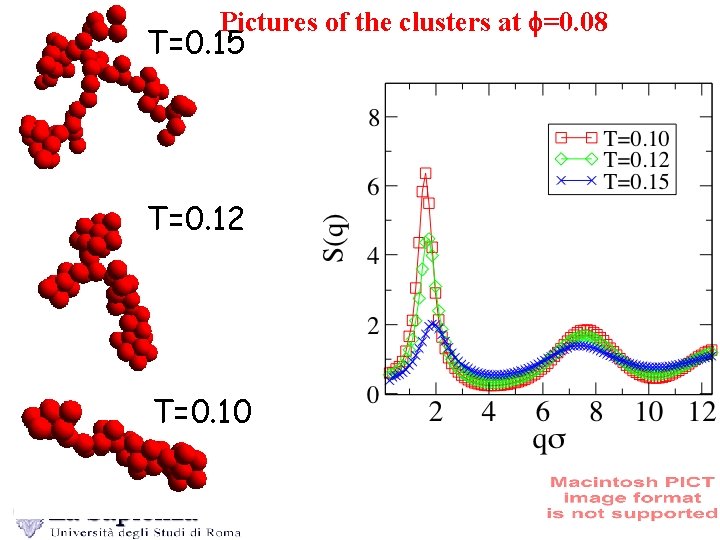
Pictures of the clusters at f=0. 08 T=0. 15 T=0. 12 T=0. 10

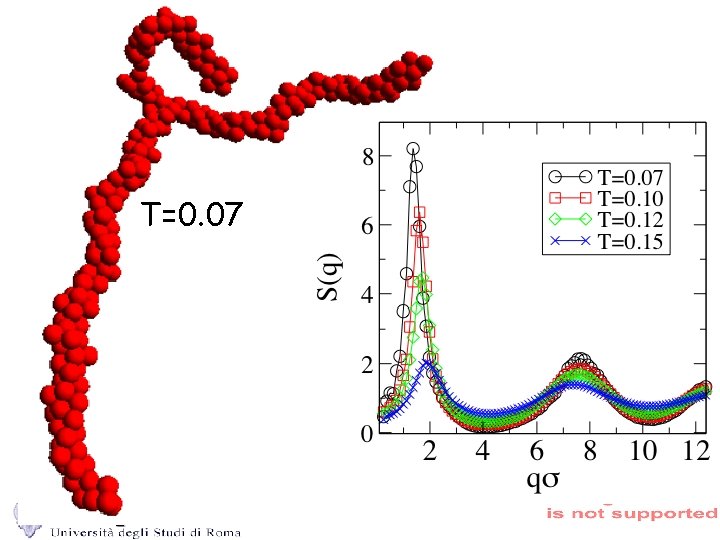
T=0. 07

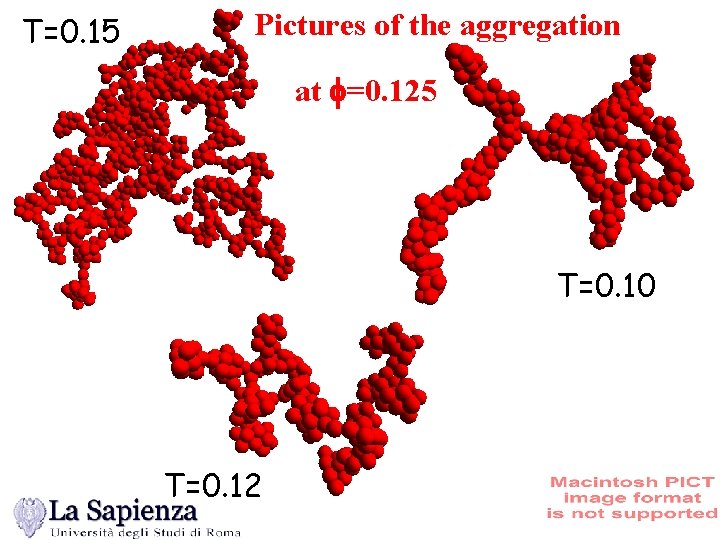
T=0. 15 Pictures of the aggregation at f=0. 125 T=0. 10 T=0. 12

T=0. 07 Cluster shape c=0. 125 A gel !

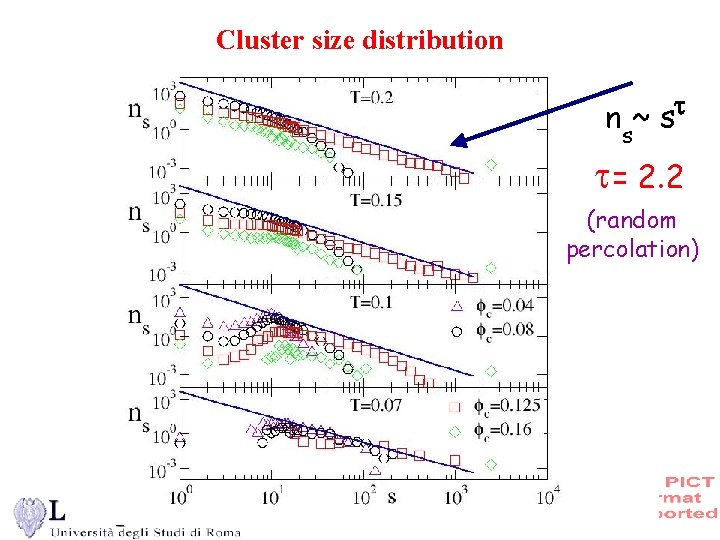
Cluster size distribution ns~ s = 2. 2 (random percolation)

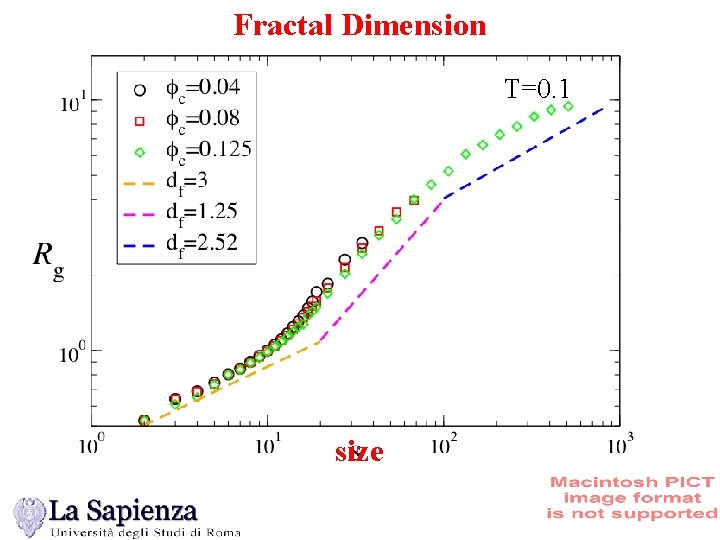
Fractal Dimension T=0. 1 size

Bond Correlation funtions stretched exponential ~0. 7 (a. u. )

Density fluctuations





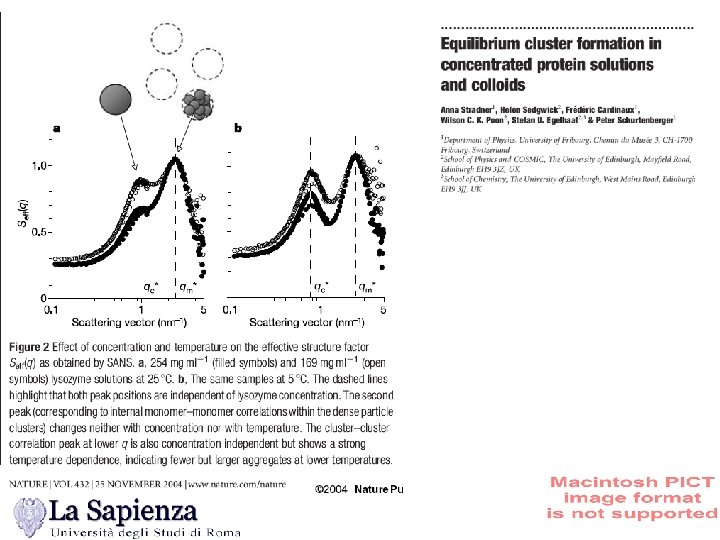
Conclusions…… Several morphologies can be generated by the competition of short-range attraction (fixing the Tscale) and the strength and length of the interaction. A new route to gelation. Continuous change from a Wigner-like glass to a gel While equilibrium would probably suggest a first order transition to a lamellar phase, arrested metastable states appear to be kinetically favored Possibility of exporting ideas developed in colloidal systems to protein systems (Schurtenberger, Chen) and, more in general to biological systems in which often one dimensional growth followed by gelation is observed.

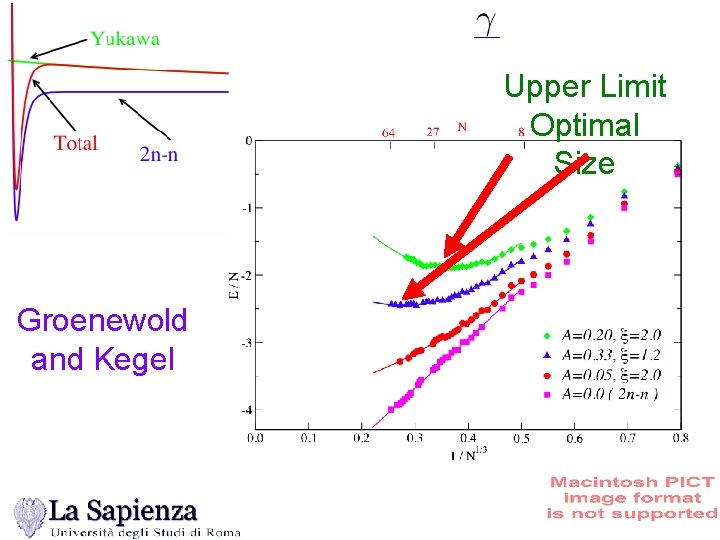
Upper Limit Optimal Size Groenewold and Kegel

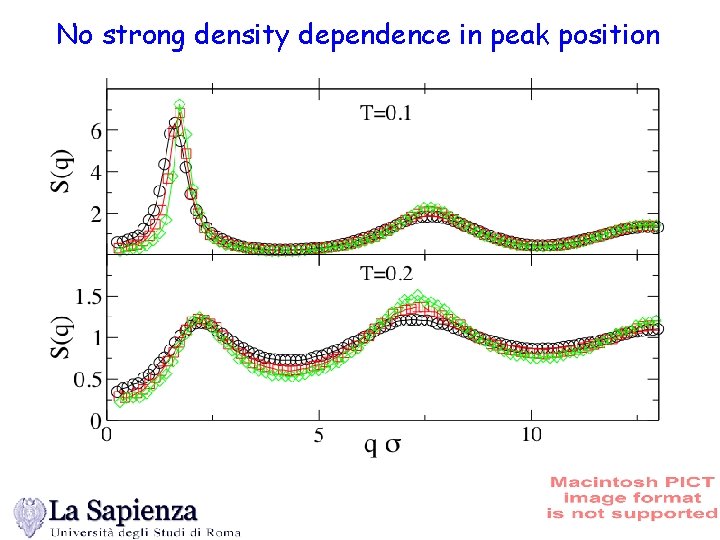
No strong density dependence in peak position

Mean square displacement


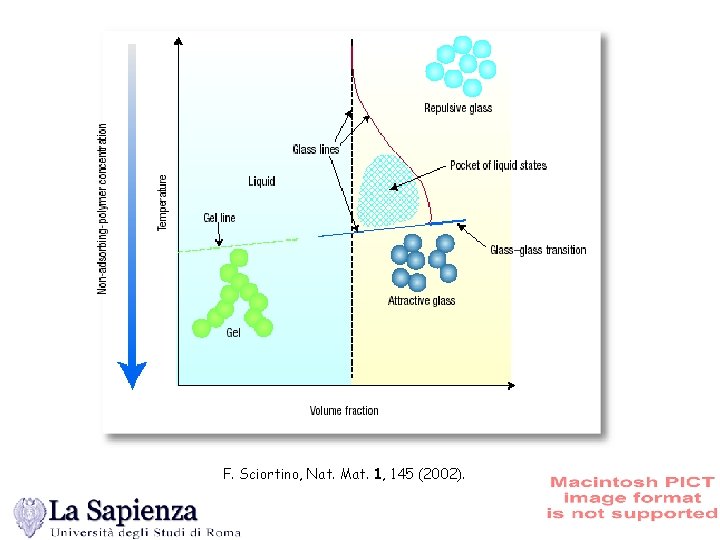
F. Sciortino, Nat. Mat. 1, 145 (2002).

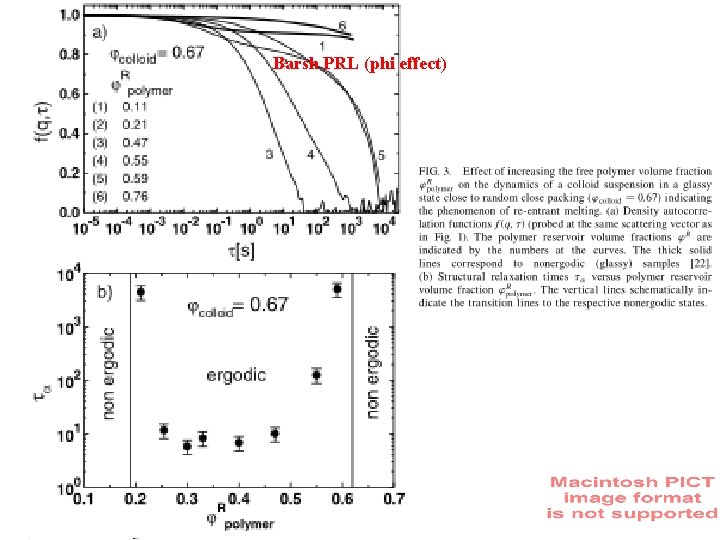
Barsh PRL (phi effect)

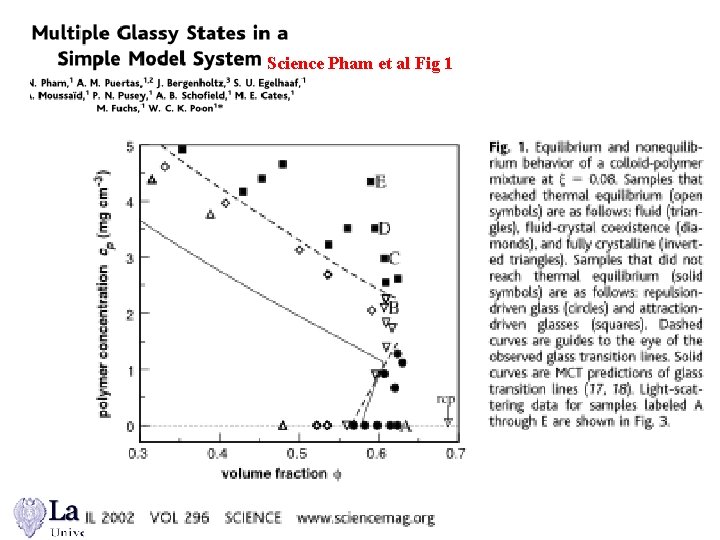
Science Pham et al Fig 1

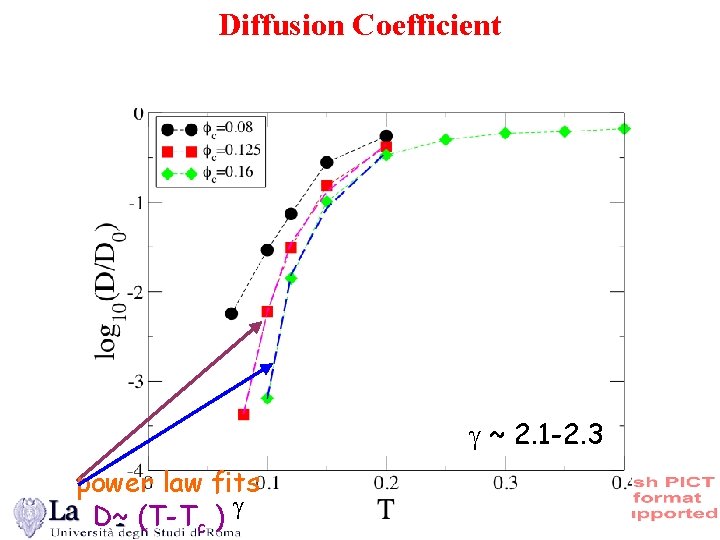
Diffusion Coefficient ~ 2. 1 -2. 3 power law fits D~ (T-Tc )


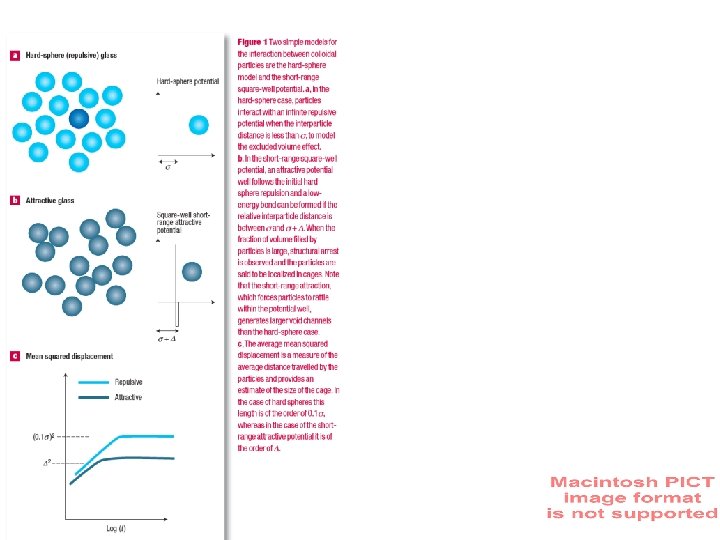
Hard Spheres Potential Mean squared displacement Hard Sphere (repulsive) glass s 2 (0. 1 s) Square-Well short range attractive D 2 Potential repulsive attractive Log(t) Attractive Glass s+D

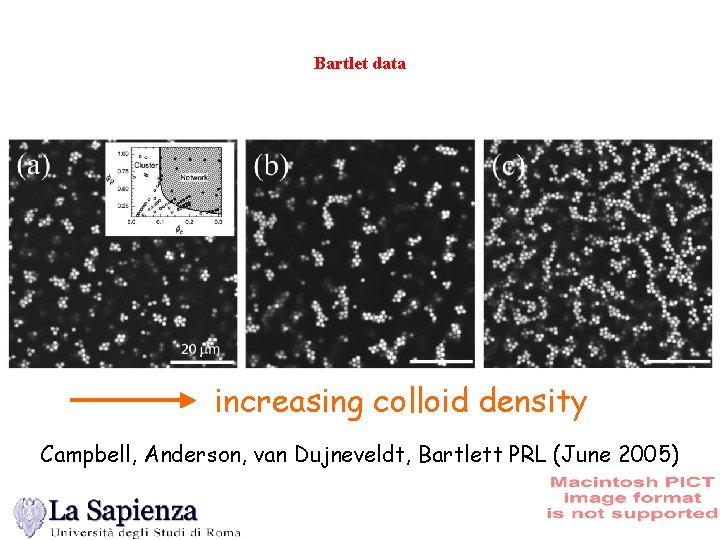
Bartlet data increasing colloid density Campbell, Anderson, van Dujneveldt, Bartlett PRL (June 2005)

Phase Diagram for Square Well (3%) Iso-diffusivity Spinodal AHS lines (Miller&Frenkel) Percolation Repulsive Line Glass Percolation Line A 3 Spinodal Liquid+Gas Attractive Glass

T=0. 15 T=0. 10
 Yukawa potential derivation
Yukawa potential derivation Yukawa potential
Yukawa potential Light cured gel enhancements should be maintained _____.
Light cured gel enhancements should be maintained _____. Differences between ointment and cream
Differences between ointment and cream Spin glasses and complexity
Spin glasses and complexity Single phase gels
Single phase gels Pastes pharmacy
Pastes pharmacy Stain free gels
Stain free gels Co solvents examples
Co solvents examples Precipitation of antibodies
Precipitation of antibodies Pempfigus
Pempfigus Cylinder transposition
Cylinder transposition Toy plural word
Toy plural word Glasses plural or singular
Glasses plural or singular Aphakic glasses disadvantages
Aphakic glasses disadvantages Disadvantages of aphakia
Disadvantages of aphakia Tich miller
Tich miller Foot plural nouns
Foot plural nouns Jinn glasses
Jinn glasses I used to wear glasses when i was at school
I used to wear glasses when i was at school Glasses also called eyeglasses (formal)
Glasses also called eyeglasses (formal) Woodshop safety glasses
Woodshop safety glasses Eye safety toolbox talk
Eye safety toolbox talk Click clack reading glasses
Click clack reading glasses Present simple affirmative negative
Present simple affirmative negative Masonic fire
Masonic fire Deadil
Deadil Lcm of 14 and 21
Lcm of 14 and 21 Rose tinted glasses an inspector calls
Rose tinted glasses an inspector calls What does atticus dropping his glasses symbolize
What does atticus dropping his glasses symbolize The woman with the glasses predicate
The woman with the glasses predicate Parisi spin glass
Parisi spin glass Prevalence of astigmatism
Prevalence of astigmatism Yep, she is who you think
Yep, she is who you think Transpose prescription examples
Transpose prescription examples The prisoner who wore glasses plot diagram
The prisoner who wore glasses plot diagram Safety glasses
Safety glasses What is this
What is this Safety glasses
Safety glasses History of glasses
History of glasses Safety glasses
Safety glasses Opvest
Opvest Glasses
Glasses I used to wear glasses
I used to wear glasses Eyring
Eyring Dave brubeck glasses
Dave brubeck glasses God view glasses
God view glasses Pronoun for her glasses
Pronoun for her glasses Christmas hyperboles
Christmas hyperboles Phet charges and charged objects investigation
Phet charges and charged objects investigation Alliteration for strong
Alliteration for strong Imagery questions
Imagery questions Which of the charges qa, qb, and qc are positively charged?
Which of the charges qa, qb, and qc are positively charged? Static electricity
Static electricity Skyward new richmond wisconsin
Skyward new richmond wisconsin The search for fractionally charged particles has
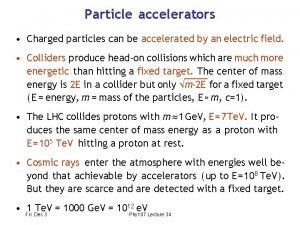
The search for fractionally charged particles has Charged particles can be accelerated by
Charged particles can be accelerated by Magnetic field and magnetic force
Magnetic field and magnetic force Chapter 6 ions charged particles in solution
Chapter 6 ions charged particles in solution Loaded words examples
Loaded words examples Why dna is negatively charged
Why dna is negatively charged