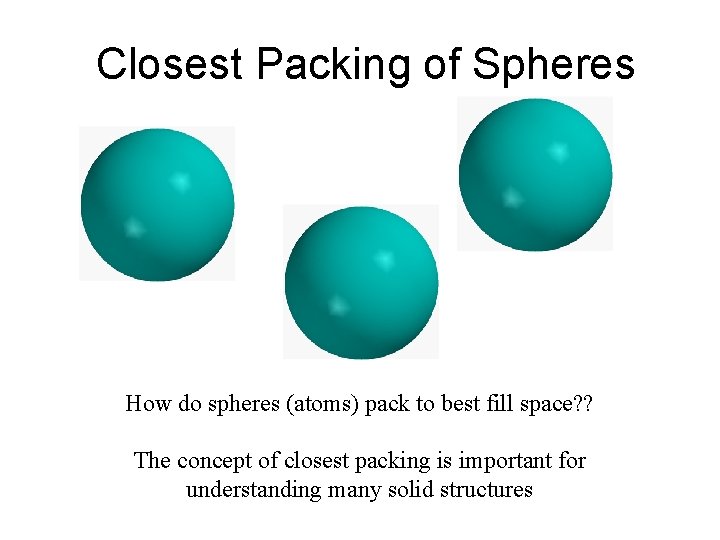
Closest Packing of Spheres How do spheres atoms

- Slides: 10

Closest Packing of Spheres How do spheres (atoms) pack to best fill space? ? The concept of closest packing is important for understanding many solid structures

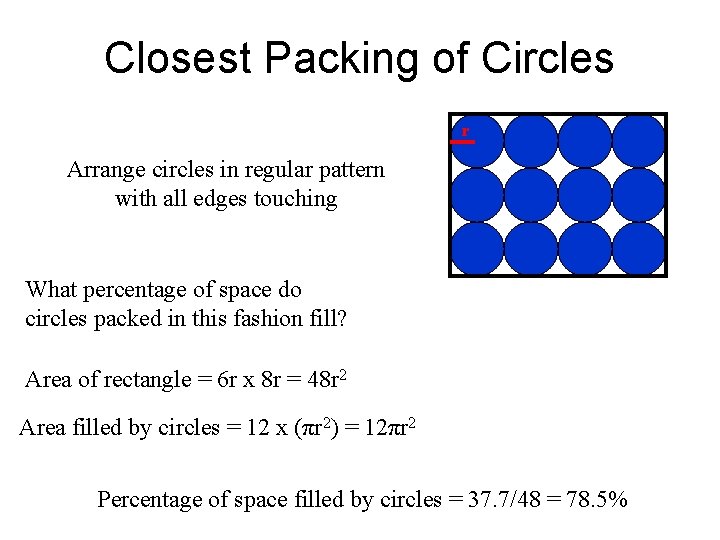
Closest Packing of Circles r Arrange circles in regular pattern with all edges touching What percentage of space do circles packed in this fashion fill? Area of rectangle = 6 r x 8 r = 48 r 2 Area filled by circles = 12 x (πr 2) = 12πr 2 Percentage of space filled by circles = 37. 7/48 = 78. 5%

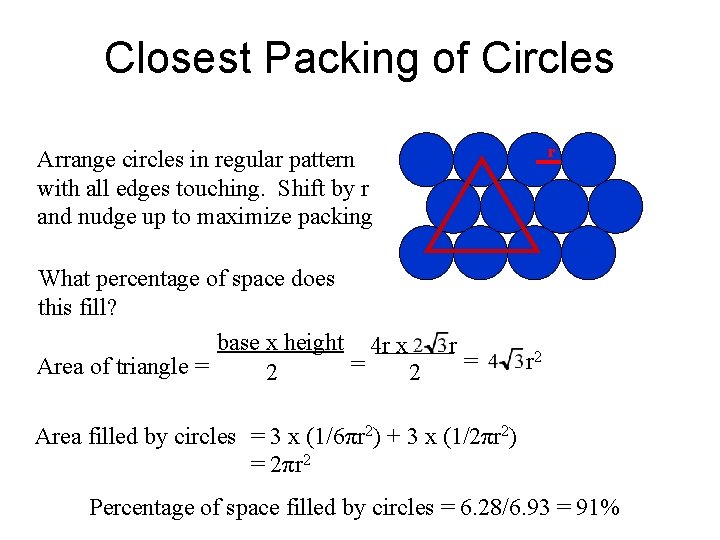
Closest Packing of Circles r Arrange circles in regular pattern with all edges touching. Shift by r and nudge up to maximize packing What percentage of space does this fill? base x height 4 r x = Area of triangle = 2 2 r = r 2 Area filled by circles = 3 x (1/6πr 2) + 3 x (1/2πr 2) = 2πr 2 Percentage of space filled by circles = 6. 28/6. 93 = 91%

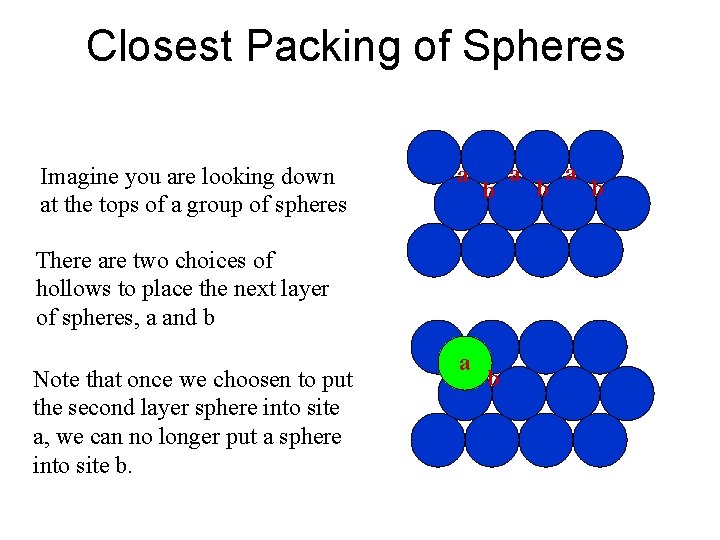
Closest Packing of Spheres Imagine you are looking down at the tops of a group of spheres a b There are two choices of hollows to place the next layer of spheres, a and b Note that once we choosen to put the second layer sphere into site a, we can no longer put a sphere into site b. a b a b

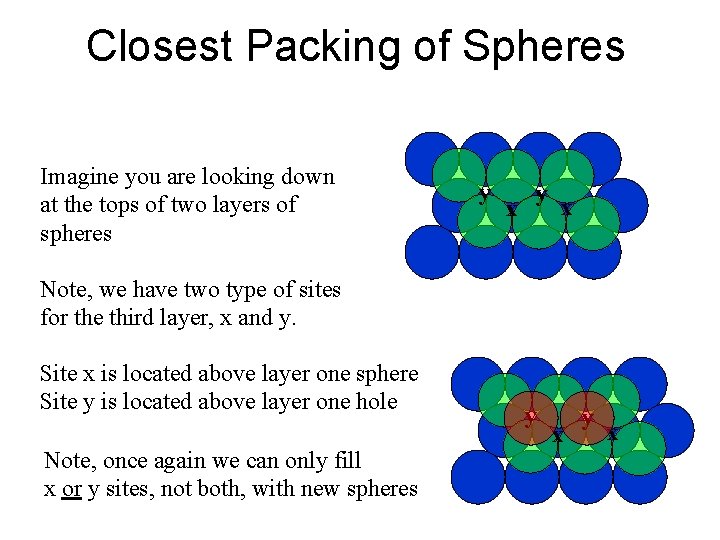
Closest Packing of Spheres Imagine you are looking down at the tops of two layers of spheres y x Note, we have two type of sites for the third layer, x and y. Site x is located above layer one sphere Site y is located above layer one hole Note, once again we can only fill x or y sites, not both, with new spheres y x

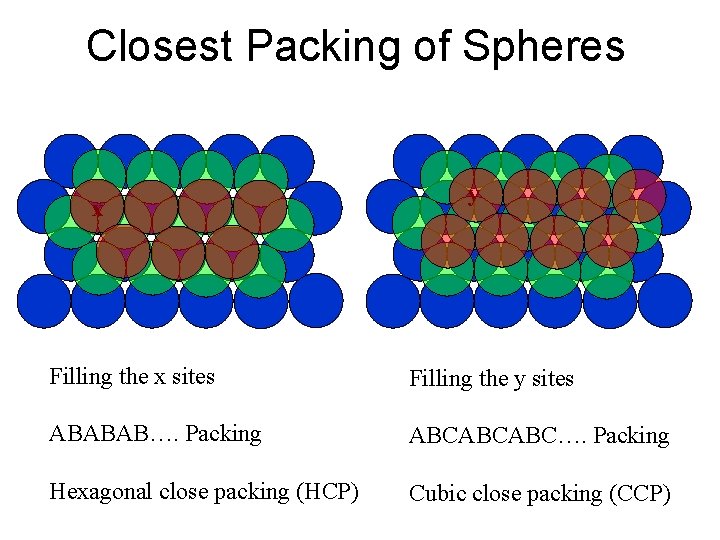
Closest Packing of Spheres x y Filling the x sites Filling the y sites ABABAB…. Packing ABCABCABC…. Packing Hexagonal close packing (HCP) Cubic close packing (CCP)

Hexagonal Close Packed Lattice

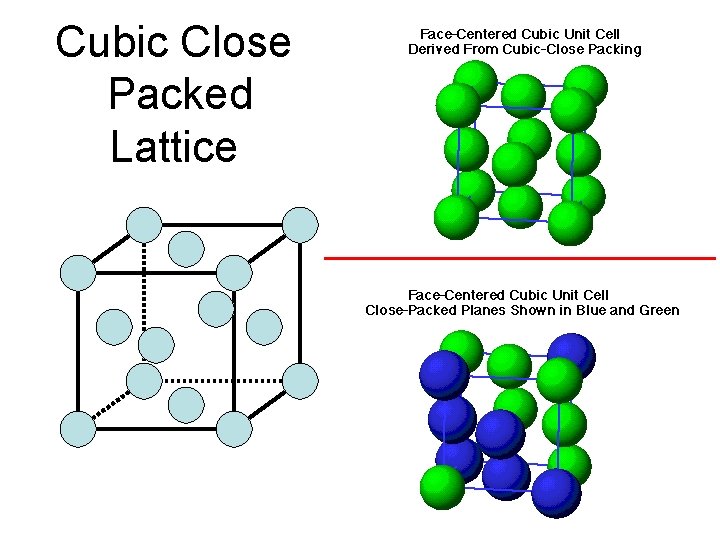
Cubic Close Packed Lattice

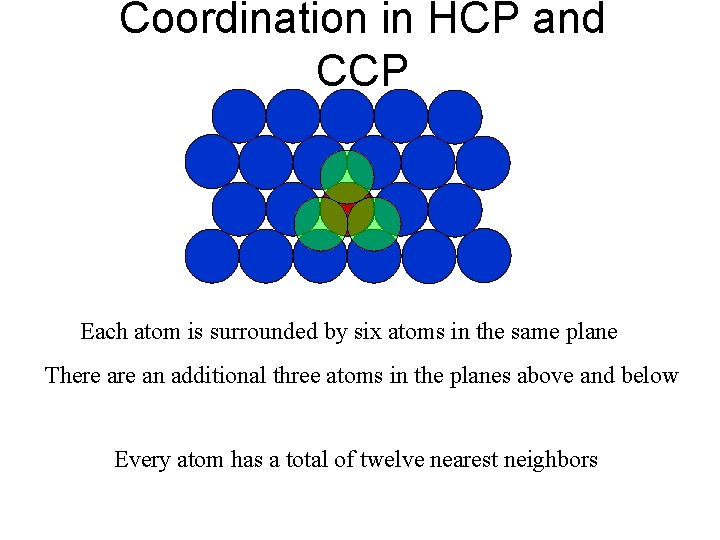
Coordination in HCP and CCP Each atom is surrounded by six atoms in the same plane There an additional three atoms in the planes above and below Every atom has a total of twelve nearest neighbors

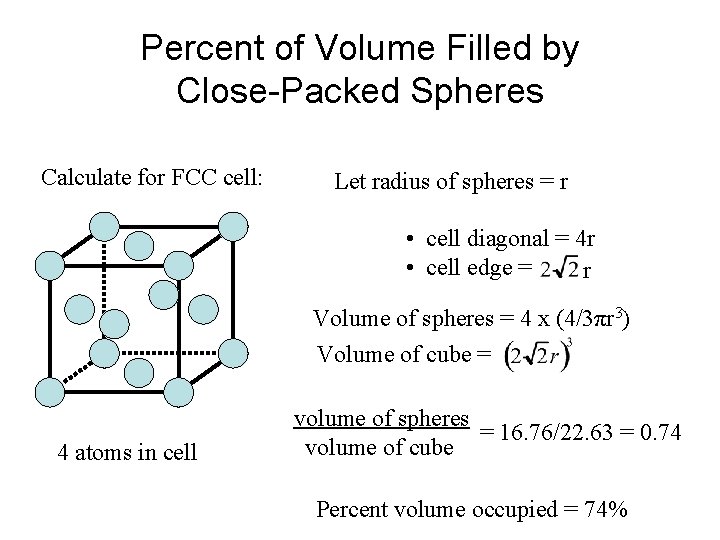
Percent of Volume Filled by Close-Packed Spheres Calculate for FCC cell: Let radius of spheres = r • cell diagonal = 4 r • cell edge = r Volume of spheres = 4 x (4/3πr 3) Volume of cube = 4 atoms in cell volume of spheres = 16. 76/22. 63 = 0. 74 volume of cube Percent volume occupied = 74%
 At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Closest packed structures
Closest packed structures Closest african country to europe
Closest african country to europe The closest algal relatives of land plants are
The closest algal relatives of land plants are Closest nested scope rule
Closest nested scope rule Vertical jaw
Vertical jaw Closest kohls
Closest kohls Cpa closest point of approach
Cpa closest point of approach Who was the closest companion of prophet muhammad
Who was the closest companion of prophet muhammad Closest nested scope rule
Closest nested scope rule Vertical dimension of occlusion
Vertical dimension of occlusion