close presentation Organic reaction maps drag and drop

- Slides: 8

close presentation Organic reaction maps drag and drop Task 1 reaction types Task 1 answers Task 2 matching structures to names Task 2 answers Task 3 reagents and conditions Task 3 answers

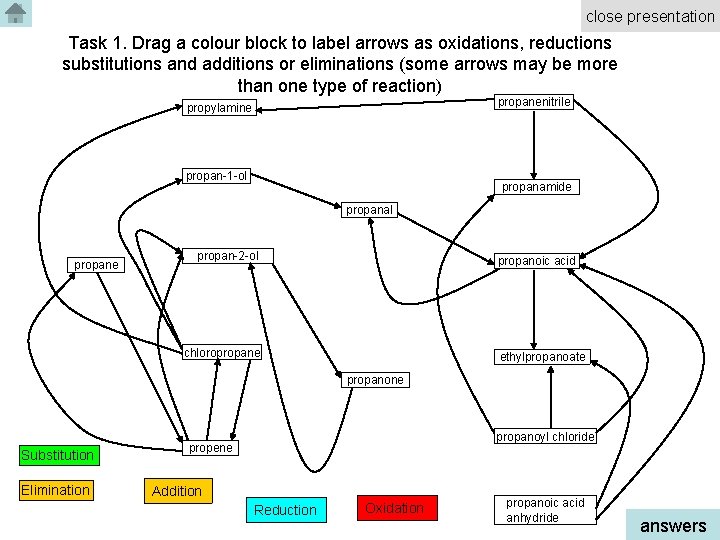
close presentation Task 1. Drag a colour block to label arrows as oxidations, reductions substitutions and additions or eliminations (some arrows may be more than one type of reaction) propanenitrile propylamine propan-1 -ol propanamide propanal propane propan-2 -ol propanoic acid chloropropane ethylpropanoate propanone Substitution Elimination propanoyl chloride propene Addition Reduction Oxidation propanoic acid anhydride answers

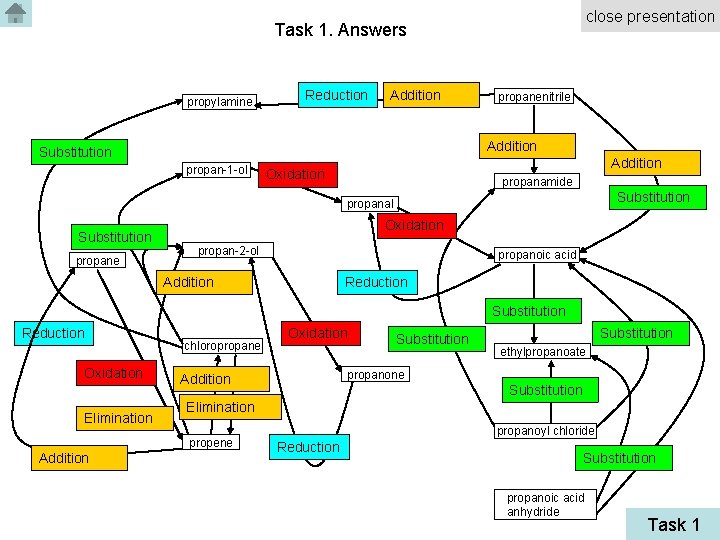
close presentation Task 1. Answers propylamine Reduction Addition propanenitrile Addition Substitution propan-1 -ol Addition Oxidation propanamide Substitution propanal Substitution propane Oxidation propan-2 -ol propanoic acid Addition Reduction Substitution Reduction Oxidation Elimination chloropropane Substitution ethylpropanoate propanone Addition Substitution Elimination propene Addition Oxidation propanoyl chloride Reduction Substitution propanoic acid anhydride Task 1

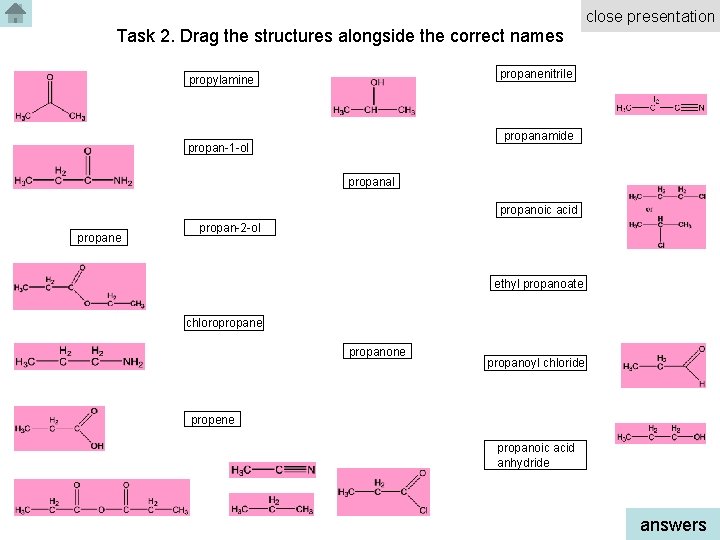
close presentation Task 2. Drag the structures alongside the correct names propanenitrile propylamine propanamide propan-1 -ol propanal propanoic acid propane propan-2 -ol ethyl propanoate chloropropane propanoyl chloride propene propanoic acid anhydride answers

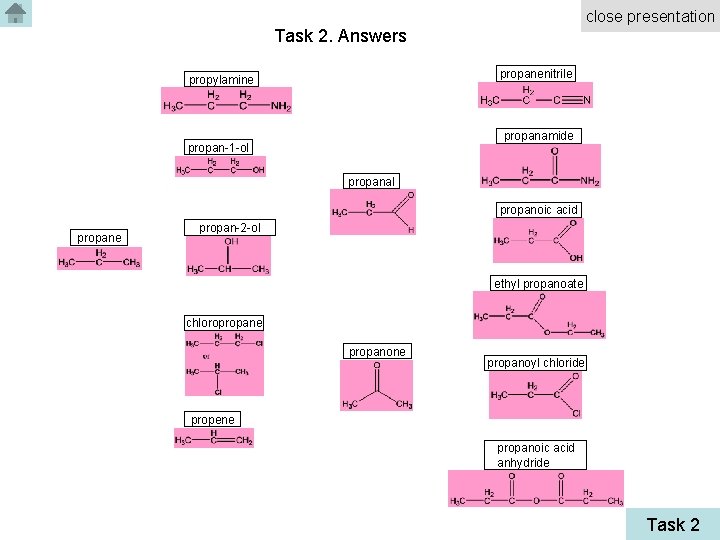
close presentation Task 2. Answers propanenitrile propylamine propanamide propan-1 -ol propanal propanoic acid propane propan-2 -ol ethyl propanoate chloropropane propanoyl chloride propene propanoic acid anhydride Task 2

close presentation Task 3. Drag and drop the correct reagents and conditions onto the correct arrows in the next slide

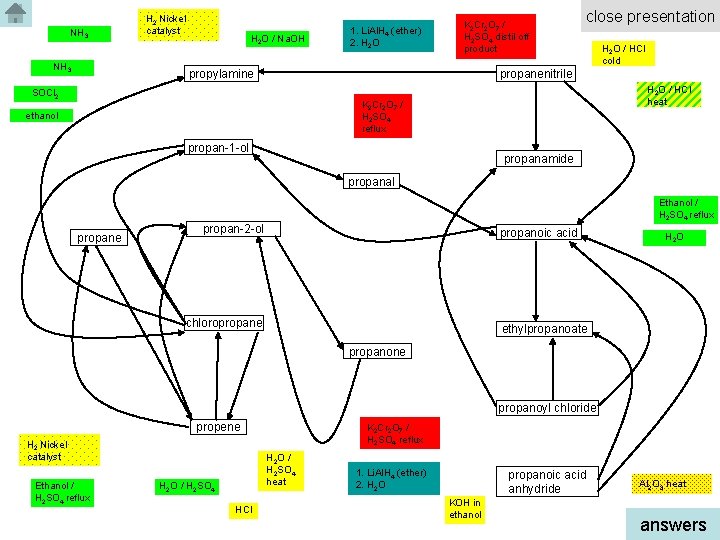
NH 3 H 2 Nickel catalyst H 2 O / Na. OH 1. Li. Al. H 4 (ether) 2. H 2 O K 2 Cr 2 O 7 / H 2 SO 4 distil off product propylamine close presentation H 2 O / HCl cold propanenitrile SOCl 2 H 2 O / HCl heat K 2 Cr 2 O 7 / H 2 SO 4 reflux ethanol propan-1 -ol propanamide propanal Ethanol / H 2 SO 4 reflux propane propan-2 -ol propanoic acid chloropropane H 2 O ethylpropanoate propanone propanoyl chloride propene H 2 Nickel catalyst Ethanol / H 2 SO 4 reflux K 2 Cr 2 O 7 / H 2 SO 4 reflux H 2 O / H 2 SO 4 heat H 2 O / H 2 SO 4 HCl 1. Li. Al. H 4 (ether) 2. H 2 O propanoic acid anhydride KOH in ethanol Al 2 O 3 heat answers

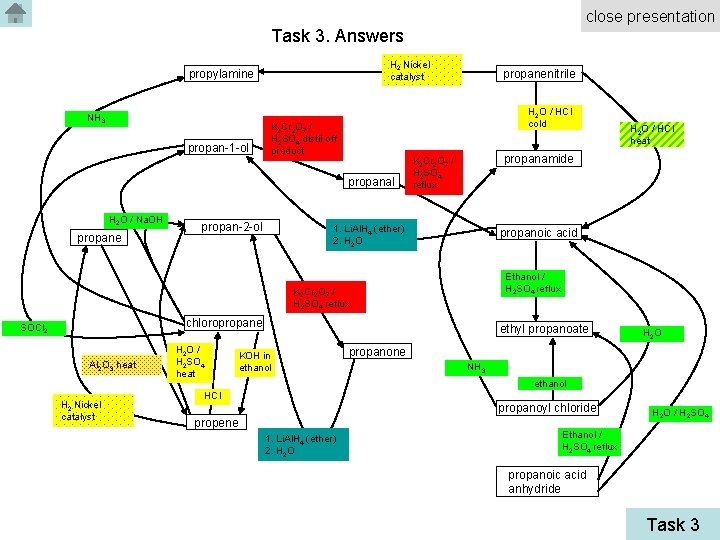
close presentation Task 3. Answers H 2 Nickel catalyst propylamine NH 3 propan-1 -ol H 2 O / HCl cold K 2 Cr 2 O 7 / H 2 SO 4 distil off product propanal H 2 O / Na. OH propane propan-2 -ol propanenitrile propanamide K 2 Cr 2 O 7 / H 2 SO 4 reflux 1. Li. Al. H 4 (ether) 2. H 2 O propanoic acid Ethanol / H 2 SO 4 reflux K 2 Cr 2 O 7 / H 2 SO 4 reflux chloropropane SOCl 2 Al 2 O 3 heat H 2 Nickel catalyst H 2 O / H 2 SO 4 heat ethyl propanoate KOH in ethanol H 2 O / HCl heat H 2 O propanone NH 3 ethanol HCl propanoyl chloride propene 1. Li. Al. H 4 (ether) 2. H 2 O / H 2 SO 4 Ethanol / H 2 SO 4 reflux propanoic acid anhydride Task 3