Clip Art courtesy of MS Office 2000 Refrigeration

- Slides: 6

Clip Art courtesy of MS Office 2000. Refrigeration and Heat Pump Systems

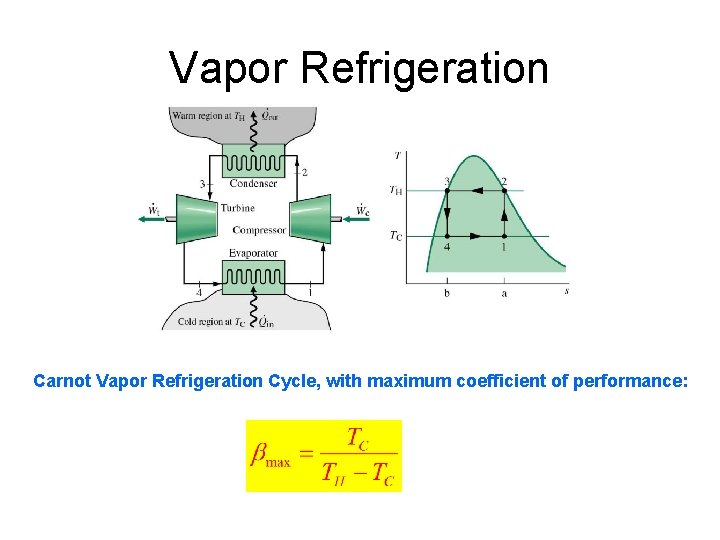
Vapor Refrigeration Carnot Vapor Refrigeration Cycle, with maximum coefficient of performance:

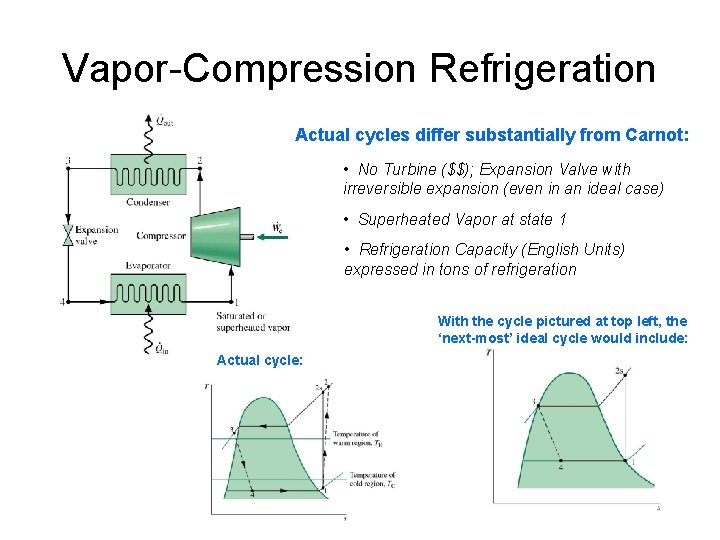
Vapor-Compression Refrigeration Actual cycles differ substantially from Carnot: • No Turbine ($$); Expansion Valve with irreversible expansion (even in an ideal case) • Superheated Vapor at state 1 • Refrigeration Capacity (English Units) expressed in tons of refrigeration With the cycle pictured at top left, the ‘next-most’ ideal cycle would include: Actual cycle:

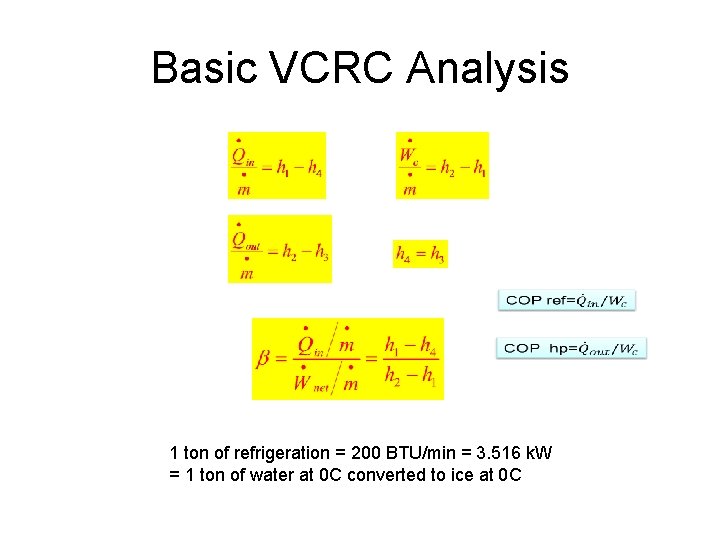
Basic VCRC Analysis 1 ton of refrigeration = 200 BTU/min = 3. 516 k. W = 1 ton of water at 0 C converted to ice at 0 C

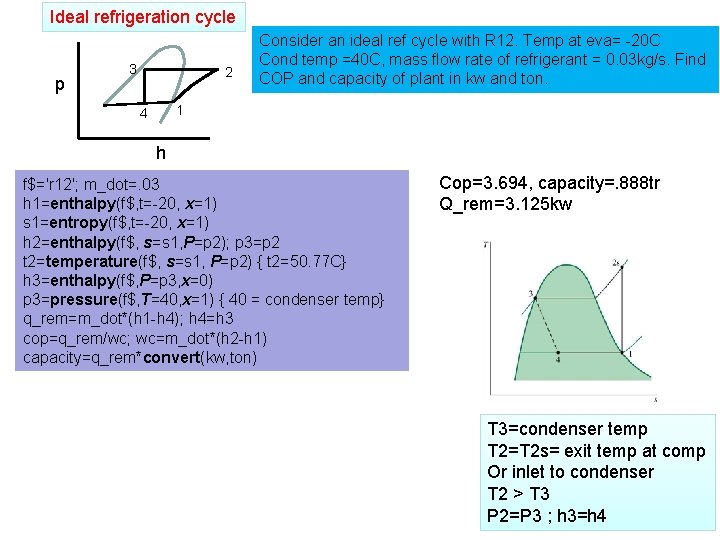
Ideal refrigeration cycle p 3 2 Consider an ideal ref cycle with R 12. Temp at eva= -20 C Cond temp =40 C, mass flow rate of refrigerant = 0. 03 kg/s. Find COP and capacity of plant in kw and ton. 1 4 h f$='r 12'; m_dot=. 03 h 1=enthalpy(f$, t=-20, x=1) s 1=entropy(f$, t=-20, x=1) h 2=enthalpy(f$, s=s 1, P=p 2); p 3=p 2 t 2=temperature(f$, s=s 1, P=p 2) { t 2=50. 77 C} h 3=enthalpy(f$, P=p 3, x=0) p 3=pressure(f$, T=40, x=1) { 40 = condenser temp} q_rem=m_dot*(h 1 -h 4); h 4=h 3 cop=q_rem/wc; wc=m_dot*(h 2 -h 1) capacity=q_rem*convert(kw, ton) Cop=3. 694, capacity=. 888 tr Q_rem=3. 125 kw T 3=condenser temp T 2=T 2 s= exit temp at comp Or inlet to condenser T 2 > T 3 P 2=P 3 ; h 3=h 4

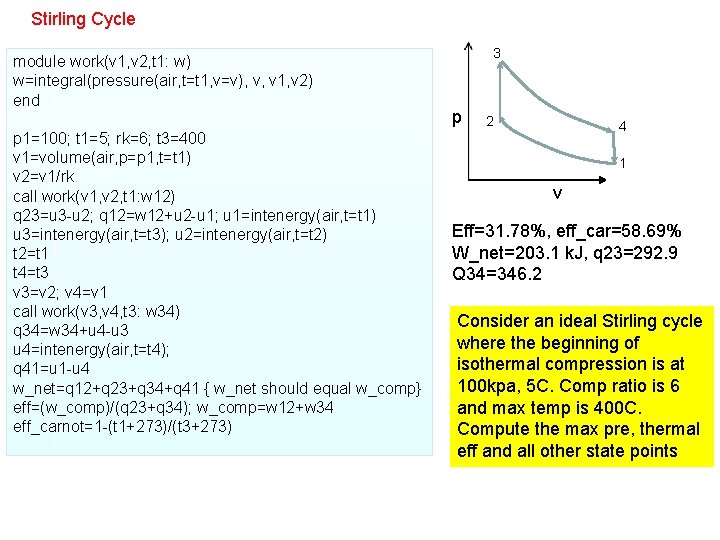
Stirling Cycle module work(v 1, v 2, t 1: w) w=integral(pressure(air, t=t 1, v=v), v, v 1, v 2) end p 1=100; t 1=5; rk=6; t 3=400 v 1=volume(air, p=p 1, t=t 1) v 2=v 1/rk call work(v 1, v 2, t 1: w 12) q 23=u 3 -u 2; q 12=w 12+u 2 -u 1; u 1=intenergy(air, t=t 1) u 3=intenergy(air, t=t 3); u 2=intenergy(air, t=t 2) t 2=t 1 t 4=t 3 v 3=v 2; v 4=v 1 call work(v 3, v 4, t 3: w 34) q 34=w 34+u 4 -u 3 u 4=intenergy(air, t=t 4); q 41=u 1 -u 4 w_net=q 12+q 23+q 34+q 41 { w_net should equal w_comp} eff=(w_comp)/(q 23+q 34); w_comp=w 12+w 34 eff_carnot=1 -(t 1+273)/(t 3+273) 3 p 2 4 1 v Eff=31. 78%, eff_car=58. 69% W_net=203. 1 k. J, q 23=292. 9 Q 34=346. 2 Consider an ideal Stirling cycle where the beginning of isothermal compression is at 100 kpa, 5 C. Comp ratio is 6 and max temp is 400 C. Compute the max pre, thermal eff and all other state points