Clinical Update Full Spectrum Treatment of Alzheimers Disease
















- Slides: 42

Clinical Update: Full Spectrum Treatment of Alzheimer’s Disease

Alzheimer’s Disease Economic Consequences ► Third most expensive disease in the U. S. ► Costs over $100 billion/year ► Further $33 billion in lost productivity and other employer costs ► 3/4 of patients admitted to residential care within 5 years of diagnosis Evans DA, Scherr PA, Smith LA, et al. Aging (Milano). 1990(Sept); 2(3): 298 -302; Ernst RL, Hay JW. Am J Public Health. 1994(Aug); 84(8): 1261 -1264; Alzheimer’s Association, 2002

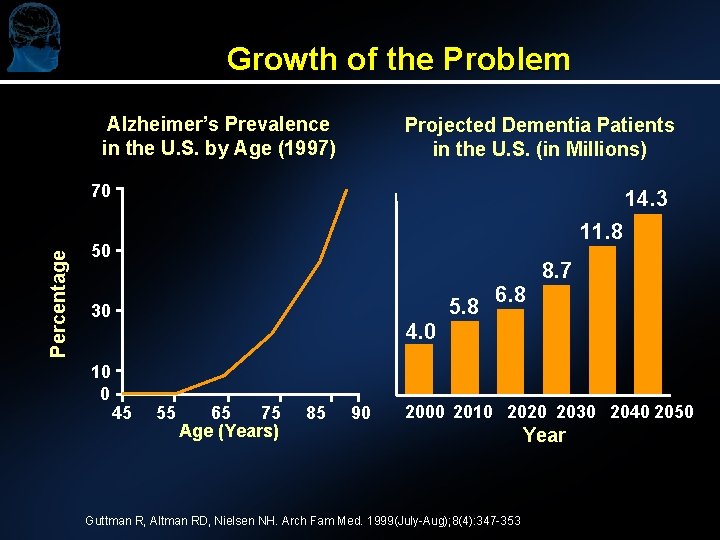
Growth of the Problem Alzheimer’s Prevalence in the U. S. by Age (1997) Projected Dementia Patients in the U. S. (in Millions) Percentage 70 14. 3 11. 8 50 5. 8 30 10 0 6. 8 8. 7 4. 0 45 55 65 75 Age (Years) 85 90 2000 2010 2020 2030 2040 2050 Guttman R, Altman RD, Nielsen NH. Arch Fam Med. 1999(July-Aug); 8(4): 347 -353 Year

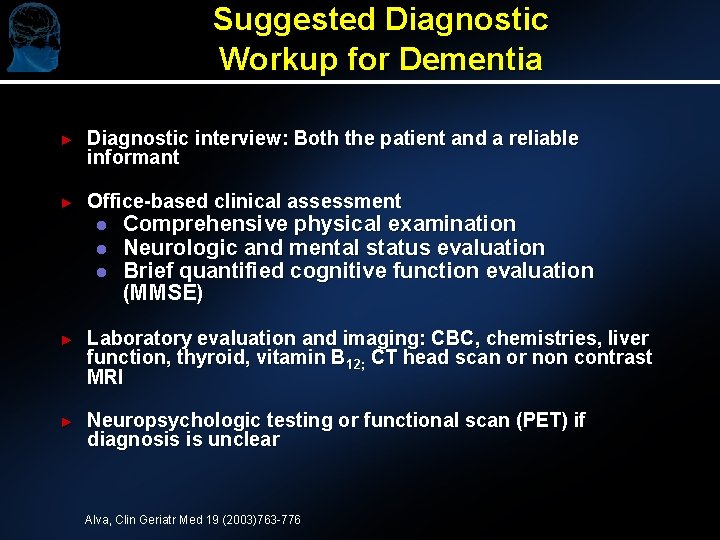
Suggested Diagnostic Workup for Dementia ► Diagnostic interview: Both the patient and a reliable informant ► Office-based clinical assessment l l l Comprehensive physical examination Neurologic and mental status evaluation Brief quantified cognitive function evaluation (MMSE) ► Laboratory evaluation and imaging: CBC, chemistries, liver function, thyroid, vitamin B 12; CT head scan or non contrast MRI ► Neuropsychologic testing or functional scan (PET) if diagnosis is unclear Alva, Clin Geriatr Med 19 (2003)763 -776

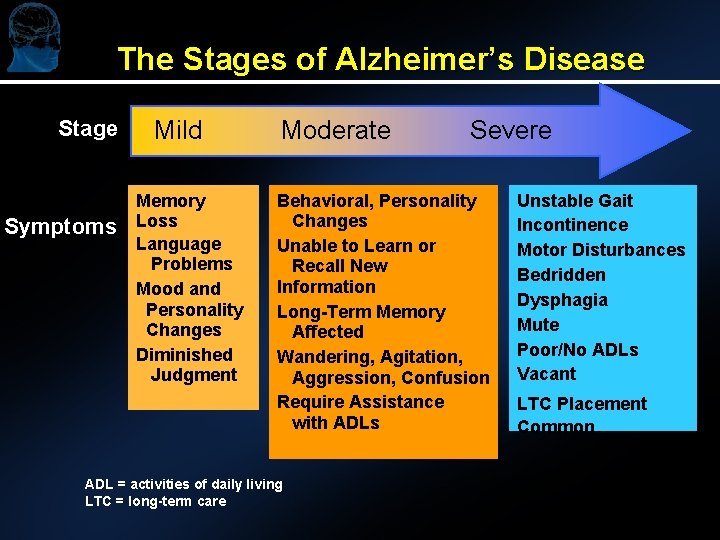
The Stages of Alzheimer’s Disease Stage Symptoms Mild Memory Loss Language Problems Mood and Personality Changes Diminished Judgment Moderate Severe Behavioral, Personality Changes Unable to Learn or Recall New Information Long-Term Memory Affected Wandering, Agitation, Aggression, Confusion Require Assistance with ADLs ADL = activities of daily living LTC = long-term care Unstable Gait Incontinence Motor Disturbances Bedridden Dysphagia Mute Poor/No ADLs Vacant LTC Placement Common

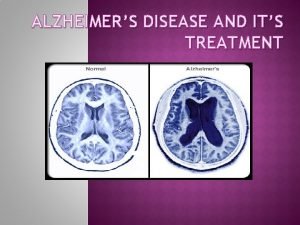
Neuropathological Changes Characteristic of AD AD Normal AP AP = amyloid plaques; NFT = neurofibrillary tangles Courtesy of George Grossberg M. D. ; St. Louis University NFT

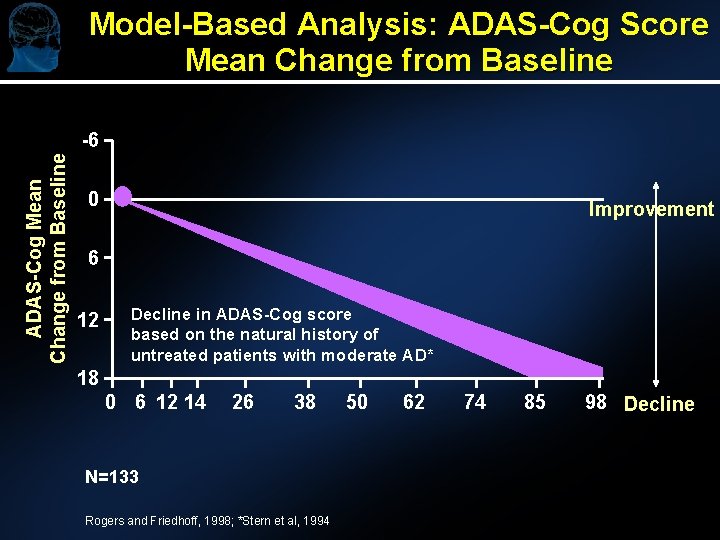
Model-Based Analysis: ADAS-Cog Score Mean Change from Baseline ADAS-Cog Mean Change from Baseline -6 0 Improvement 6 12 Decline in ADAS-Cog score based on the natural history of untreated patients with moderate AD* 18 0 6 12 14 26 38 N=133 Rogers and Friedhoff, 1998; *Stern et al, 1994 50 62 74 85 98 Decline

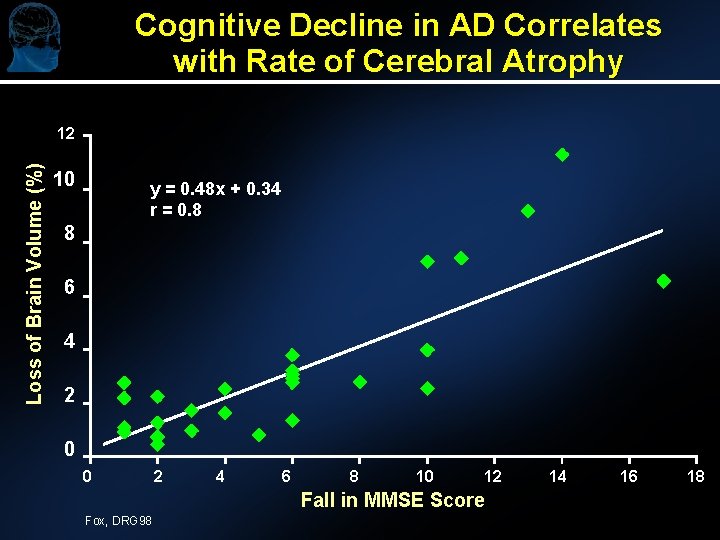
Cognitive Decline in AD Correlates with Rate of Cerebral Atrophy Loss of Brain Volume (%) 12 10 y = 0. 48 x + 0. 34 r = 0. 8 8 6 4 2 0 0 2 4 6 8 10 12 Fall in MMSE Score Fox, DRG 98 14 16 18

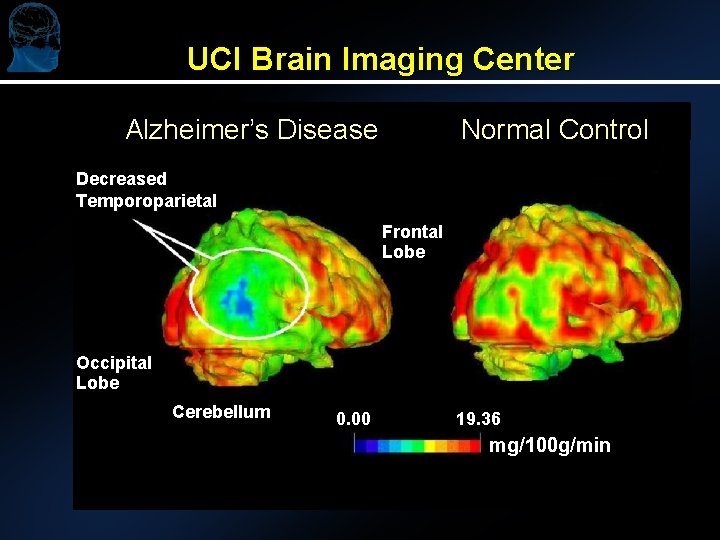
UCI Brain Imaging Center Alzheimer’s Disease Normal Control Decreased Temporoparietal Frontal Lobe Occipital Lobe Cerebellum 0. 00 19. 36 mg/100 g/min

Management of the AD Patient Treatment Goals Source: ► Maintain quality of life ► Maximize function ► Stabilize cognition ► Treat mood and behavior problems ► Ease caregiver burden Cefalu C, Grossberg GT. Diagnosis and Management of Dementia. American Family Physician Monograph, No. 2. Leawood, Kan: American Academy of Family Physicians; 2001.

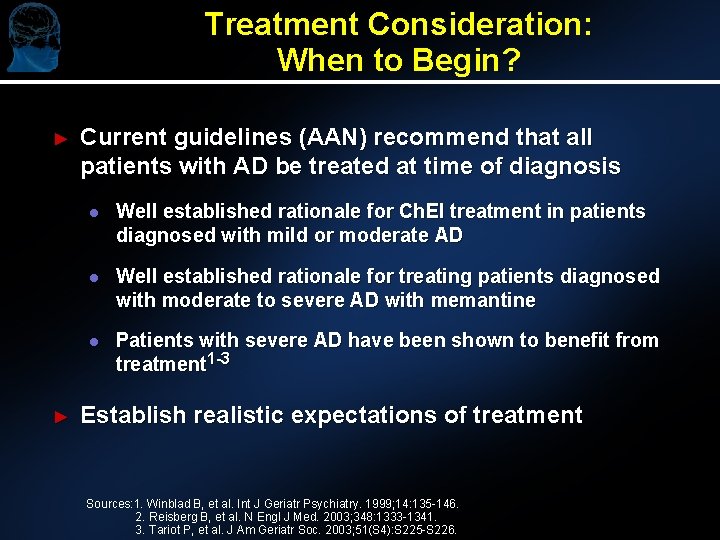
Treatment Consideration: When to Begin? ► ► Current guidelines (AAN) recommend that all patients with AD be treated at time of diagnosis l Well established rationale for Ch. EI treatment in patients diagnosed with mild or moderate AD l Well established rationale for treating patients diagnosed with moderate to severe AD with memantine l Patients with severe AD have been shown to benefit from treatment 1 -3 Establish realistic expectations of treatment Sources: 1. Winblad B, et al. Int J Geriatr Psychiatry. 1999; 14: 135 -146. 2. Reisberg B, et al. N Engl J Med. 2003; 348: 1333 -1341. 3. Tariot P, et al. J Am Geriatr Soc. 2003; 51(S 4): S 225 -S 226.

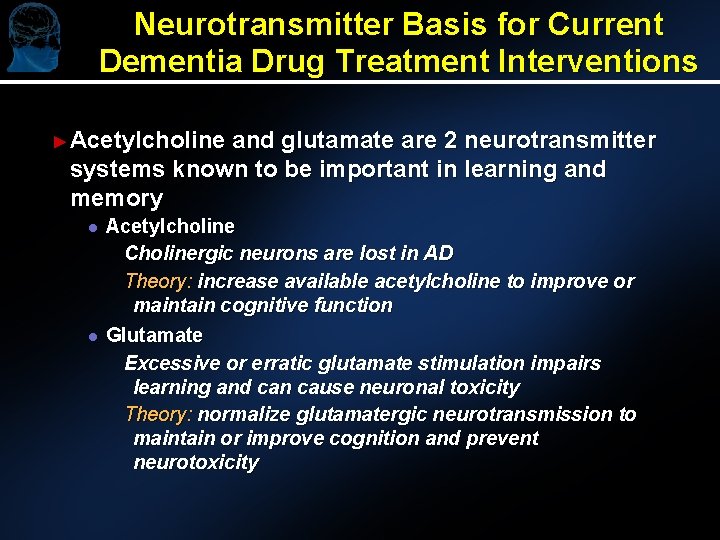
Neurotransmitter Basis for Current Dementia Drug Treatment Interventions ► Acetylcholine and glutamate are 2 neurotransmitter systems known to be important in learning and memory l l Acetylcholine Cholinergic neurons are lost in AD Theory: increase available acetylcholine to improve or maintain cognitive function Glutamate Excessive or erratic glutamate stimulation impairs learning and can cause neuronal toxicity Theory: normalize glutamatergic neurotransmission to maintain or improve cognition and prevent neurotoxicity

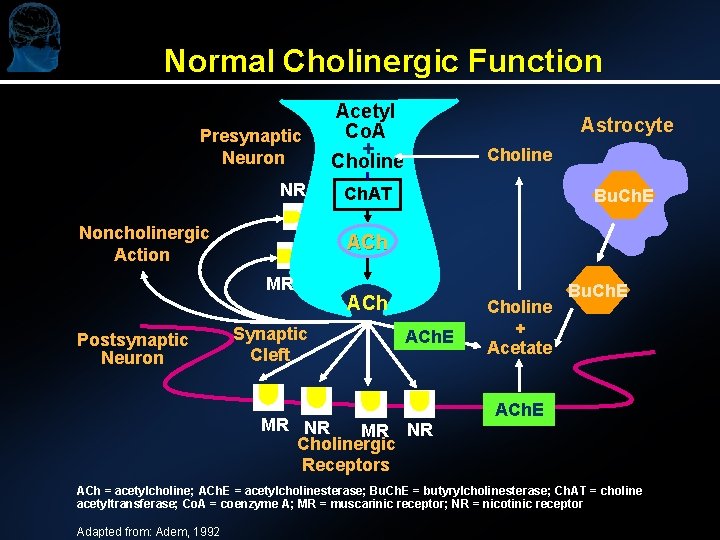
Normal Cholinergic Function Presynaptic Neuron NR Noncholinergic Action Astrocyte Choline Ch. AT Bu. Ch. E ACh MR Postsynaptic Neuron Acetyl Co. A + Choline Synaptic Cleft ACh. E MR NR Cholinergic Receptors Choline + Acetate Bu. Ch. E ACh = acetylcholine; ACh. E = acetylcholinesterase; Bu. Ch. E = butyrylcholinesterase; Ch. AT = choline acetyltransferase; Co. A = coenzyme A; MR = muscarinic receptor; NR = nicotinic receptor Adapted from: Adem, 1992

Pharmacotherapy for Mild to Moderate Alzheimer’s Disease FDA Approved: ► Cholinesterase inhibitors (Ch. EIs) l Tacrine l Donepezil l Galantamine l Rivastigmine l Monotherapy as standard treatment New Developments in Mild AD: ► NMDA-receptor antagonist (memantine) – Monotherapy l Combination Therapy

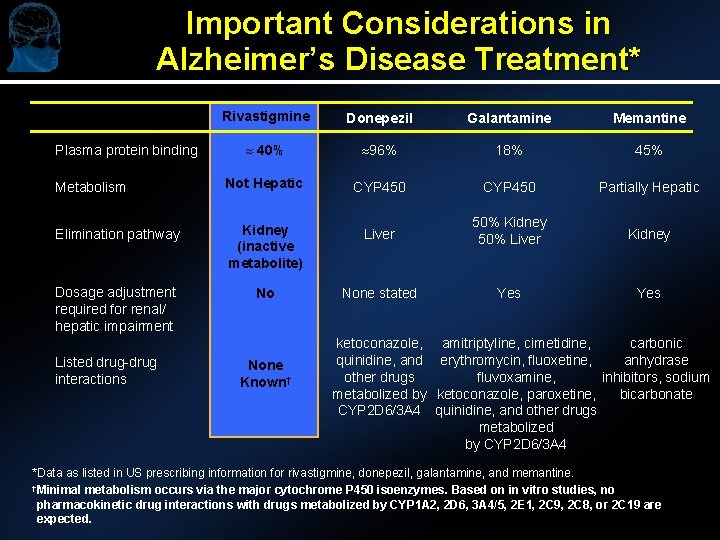
Important Considerations in Alzheimer’s Disease Treatment* Rivastigmine Donepezil Galantamine Memantine » 40% » 96% 18% 45% Metabolism Not Hepatic CYP 450 Partially Hepatic Elimination pathway Kidney (inactive metabolite) Liver 50% Kidney 50% Liver Kidney Dosage adjustment required for renal/ hepatic impairment No None stated Yes Plasma protein binding Listed drug-drug interactions None Known† ketoconazole, amitriptyline, cimetidine, carbonic quinidine, and erythromycin, fluoxetine, anhydrase other drugs fluvoxamine, inhibitors, sodium metabolized by ketoconazole, paroxetine, bicarbonate CYP 2 D 6/3 A 4 quinidine, and other drugs metabolized by CYP 2 D 6/3 A 4 *Data as listed in US prescribing information for rivastigmine, donepezil, galantamine, and memantine. †Minimal metabolism occurs via the major cytochrome P 450 isoenzymes. Based on in vitro studies, no pharmacokinetic drug interactions with drugs metabolized by CYP 1 A 2, 2 D 6, 3 A 4/5, 2 E 1, 2 C 9, 2 C 8, or 2 C 19 are expected.

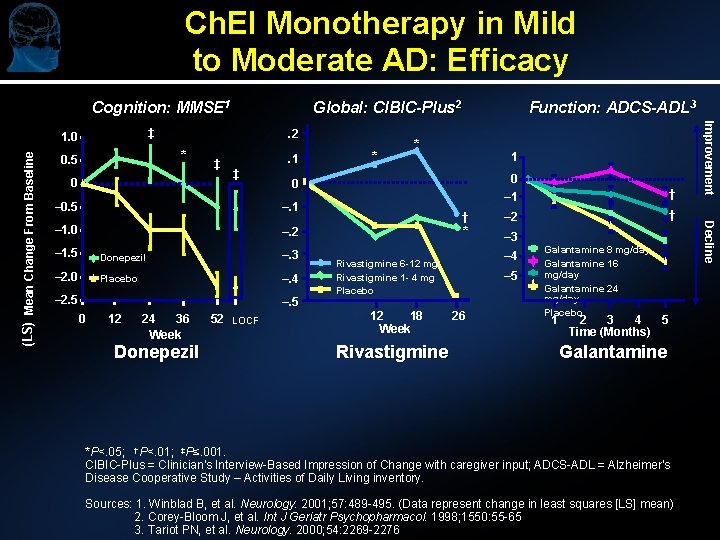
Ch. EI Monotherapy in Mild to Moderate AD: Efficacy Cognition: MMSE 1. 2 * 0. 5 0 ‡ . 1 ‡ – 1. 0 –. 2 – 1. 5 Donepezil –. 3 – 2. 0 Placebo –. 4 –. 5 12 24 36 Week Donepezil 52 LOCF 1 0 – 1 † † – 2 † * – 3 – 4 Rivastigmine 6 -12 mg Rivastigmine 1 - 4 mg Placebo 12 18 Week Rivastigmine – 5 26 Galantamine 8 mg/day Galantamine 16 mg/day Galantamine 24 mg/day Placebo 1 2 3 4 5 Time (Months) Galantamine *P<. 05; †P<. 01; ‡P≤. 001. CIBIC-Plus = Clinician's Interview-Based Impression of Change with caregiver input; ADCS-ADL = Alzheimer's Disease Cooperative Study – Activities of Daily Living inventory. Sources: 1. Winblad B, et al. Neurology. 2001; 57: 489 -495. (Data represent change in least squares [LS] mean) 2. Corey-Bloom J, et al. Int J Geriatr Psychopharmacol. 1998; 1550: 55 -65 3. Tariot PN, et al. Neurology. 2000; 54: 2269 -2276 Decline –. 1 0 * 0 – 0. 5 – 2. 5 * Function: ADCS-ADL 3 Improvement ‡ 1. 0 (LS) Mean Change From Baseline Global: CIBIC-Plus 2

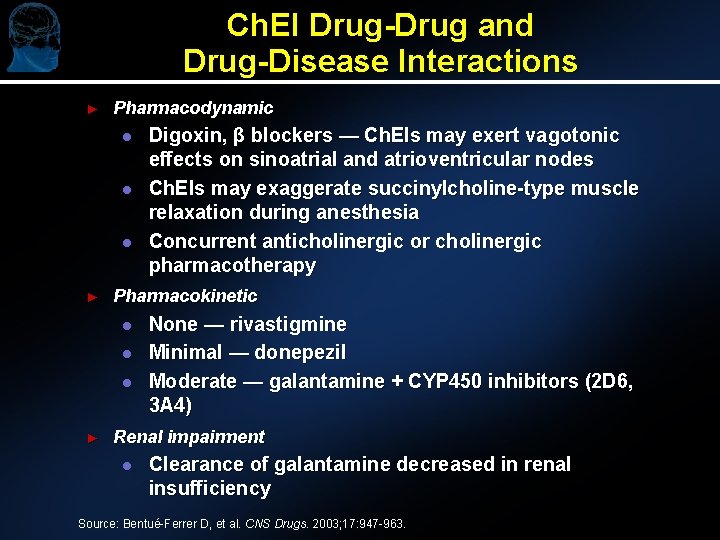
Ch. EI Drug-Drug and Drug-Disease Interactions ► Pharmacodynamic l l l ► Pharmacokinetic l l l ► Digoxin, β blockers — Ch. EIs may exert vagotonic effects on sinoatrial and atrioventricular nodes Ch. EIs may exaggerate succinylcholine-type muscle relaxation during anesthesia Concurrent anticholinergic or cholinergic pharmacotherapy None — rivastigmine Minimal — donepezil Moderate — galantamine + CYP 450 inhibitors (2 D 6, 3 A 4) Renal impairment l Clearance of galantamine decreased in renal insufficiency Source: Bentué-Ferrer D, et al. CNS Drugs. 2003; 17: 947 -963.

Treatment Consideration: When to Increase Dose or Switch Agents? ► Dose escalation may need to be slower than suggested in Physicians’ Desk Reference ► Side effects to treatment are justifiable reasons to switch ► Typically, switching Ch. EIs can be done without washout period and with shorter titration periods ► Evidence shows that memantine, a noncholinergic agent, is effective as monotherapy and in combination therapy with a Ch. EI 1, 2 Sources: 1. Reisberg B, et al. N Engl J Med. 2003; 348: 1333 -1341. 2. Tariot P, et al. JAMA. 2004; 291: 317 -324.

Treatment Consideration: When to Stop? ► May not tolerate cholinergic side effects despite slow and careful escalation ► When medication is prescribed, give it time to work; gauge different domains ► Establishing benefit in an individual patient may be influenced by their staging ► Studies suggest that most subjects benefit and that long-term treatment is useful ► May see some deterioration when medication is stopped

Memantine in Mild to Moderate AD: Clinical Trials ► ► Monotherapy Trials: l US 24 -week trial l European 24 -week trial Statistically significant advantage of memantine over placebo at end point on cognitive and global measures Numerical advantage at end point for memantine (not statistically significant) over placebo for cognitive and global measures Combination Therapy Trials: l US 24 -week trial of patients on stable Ch. EI therapy Numerical advantage at end point of memantine over placebo for cognitive, functional, and global measures (not statistically significant) Source: Peskind E, et al. Presented at the 8 th Congress of the European Federation of Neurological Societies; September 4 -7, 2004; Paris, France.

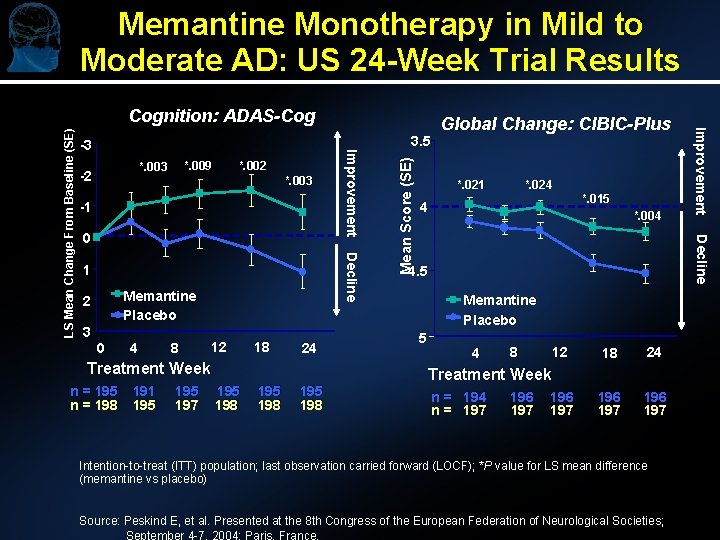
Memantine Monotherapy in Mild to Moderate AD: US 24 -Week Trial Results *. 002 *. 003 -1 Decline 1 Memantine Placebo 2 3 0 4 8 12 18 24 Treatment Week n = 195 n = 198 191 195 197 195 198 *. 021 *. 024 4 *. 015 *. 004 Decline 0 Mean Score (SE) *. 009 *. 003 -2 3. 5 Global Change: CIBIC-Plus 4. 5 Memantine Placebo 5 Improvement -3 Improvement LS Mean Change From Baseline (SE) Cognition: ADAS-Cog 4 8 12 18 24 196 197 Treatment Week n = 194 n = 197 196 197 Intention-to-treat (ITT) population; last observation carried forward (LOCF); *P value for LS mean difference (memantine vs placebo) Source: Peskind E, et al. Presented at the 8 th Congress of the European Federation of Neurological Societies; September 4 -7, 2004; Paris, France.

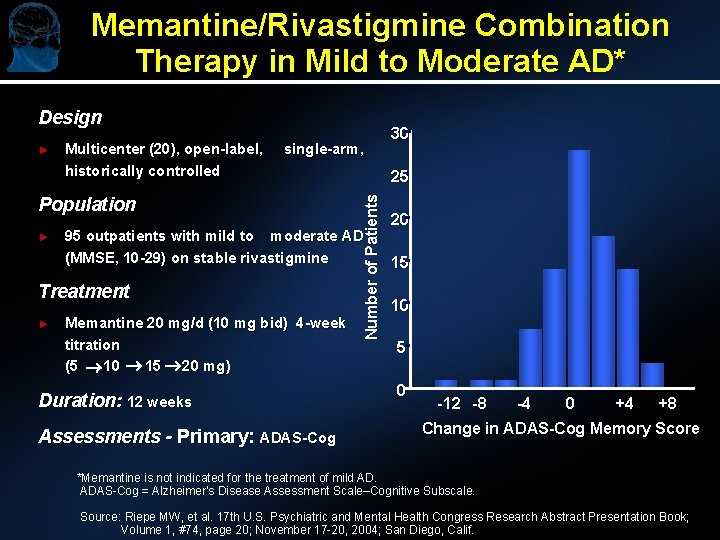
Memantine/Rivastigmine Combination Therapy in Mild to Moderate AD* Design Multicenter (20), open-label, historically controlled single-arm, Population ► 95 outpatients with mild to moderate AD (MMSE, 10 -29) on stable rivastigmine Treatment ► 25 Number of Patients ► Memantine 20 mg/d (10 mg bid) 4 -week titration (5 10 15 20 mg) Duration: 12 weeks Assessments - Primary: ADAS-Cog 30 20 15 10 5 0 -12 -8 -4 0 +4 +8 Change in ADAS-Cog Memory Score *Memantine is not indicated for the treatment of mild AD. ADAS-Cog = Alzheimer’s Disease Assessment Scale–Cognitive Subscale. Source: Riepe MW, et al. 17 th U. S. Psychiatric and Mental Health Congress Research Abstract Presentation Book; Volume 1, #74, page 20; November 17 -20, 2004; San Diego, Calif.

Memantine Adverse Events ► No clinically relevant differences between memantine- and placebo-treated groups were observed in: l l Adverse event profile Vital signs values Laboratory parameters ECG values ► Memantine l l at a dosage of 20 mg/d Exhibits a safety profile similar to that of placebo Is well tolerated and safe for the treatment of patients with AD

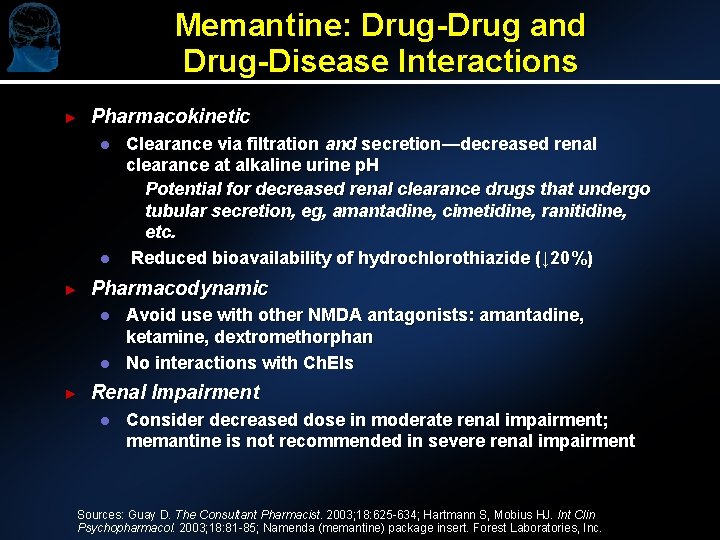
Memantine: Drug-Drug and Drug-Disease Interactions ► Pharmacokinetic l l ► Pharmacodynamic l l ► Clearance via filtration and secretion—decreased renal clearance at alkaline urine p. H Potential for decreased renal clearance drugs that undergo tubular secretion, eg, amantadine, cimetidine, ranitidine, etc. Reduced bioavailability of hydrochlorothiazide (↓ 20%) Avoid use with other NMDA antagonists: amantadine, ketamine, dextromethorphan No interactions with Ch. EIs Renal Impairment l Consider decreased dose in moderate renal impairment; memantine is not recommended in severe renal impairment Sources: Guay D. The Consultant Pharmacist. 2003; 18: 625 -634; Hartmann S, Mobius HJ. Int Clin Psychopharmacol. 2003; 18: 81 -85; Namenda (memantine) package insert. Forest Laboratories, Inc.

Behavioral Symptoms in AD ► Common ► Occur early in the disease ► May be part of the disease prodrome ► Symptoms emerge as disease progresses ► Once present, symptoms tend to persist ► Multiple types of symptoms that may occur simultaneously (eg, hallucinations, delusions, depression, euphoria, agitation, aggression, abnormal vocalization, wandering, overactivity, sexual disinhibition, sleep disturbances, and apathy)

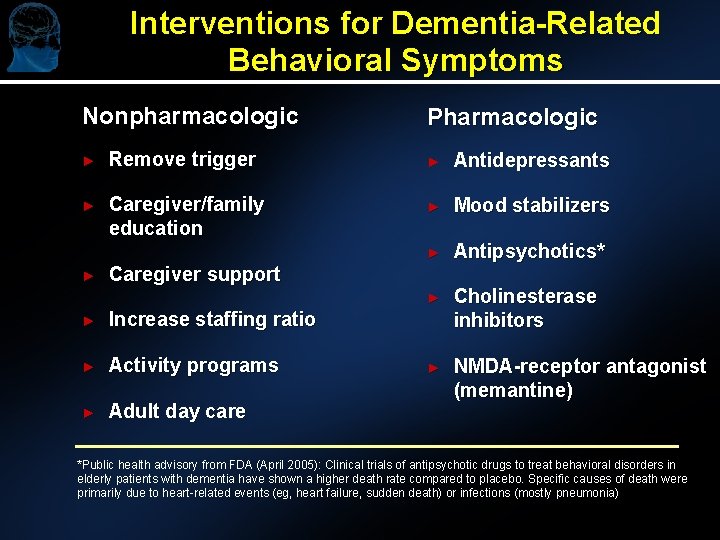
Interventions for Dementia-Related Behavioral Symptoms Nonpharmacologic Pharmacologic ► Remove trigger ► Antidepressants ► Caregiver/family education ► Mood stabilizers ► Antipsychotics* ► Cholinesterase inhibitors ► NMDA-receptor antagonist (memantine) ► Caregiver support ► Increase staffing ratio ► Activity programs ► Adult day care *Public health advisory from FDA (April 2005): Clinical trials of antipsychotic drugs to treat behavioral disorders in elderly patients with dementia have shown a higher death rate compared to placebo. Specific causes of death were primarily due to heart-related events (eg, heart failure, sudden death) or infections (mostly pneumonia)

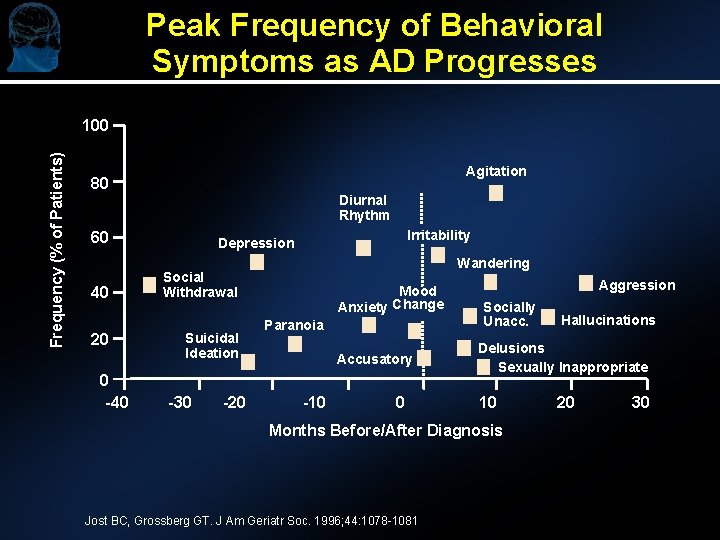
Peak Frequency of Behavioral Symptoms as AD Progresses Frequency (% of Patients) 100 Agitation 80 Diurnal Rhythm 60 40 20 0 -40 Irritability Depression Wandering Social Withdrawal Suicidal Ideation -30 -20 Mood Anxiety Change Paranoia Accusatory -10 0 Aggression Socially Unacc. Delusions Sexually Inappropriate 10 Months Before/After Diagnosis Jost BC, Grossberg GT. J Am Geriatr Soc. 1996; 44: 1078 -1081 Hallucinations 20 30

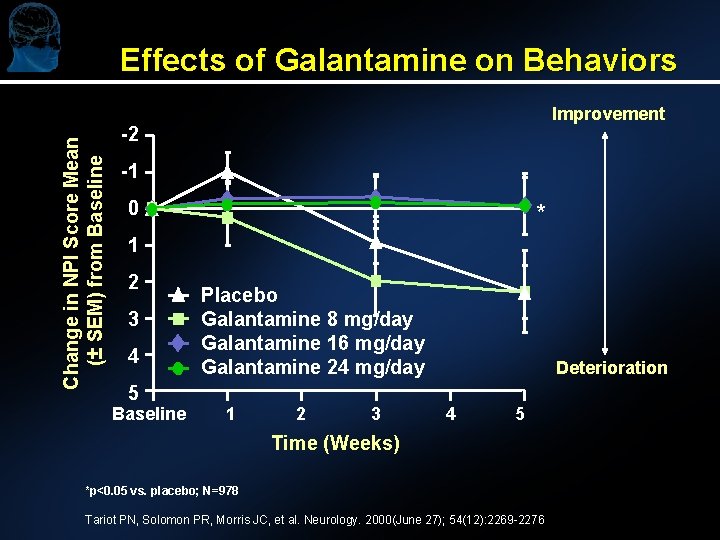
Effects of Galantamine on Behaviors Change in NPI Score Mean (± SEM) from Baseline Improvement -2 -1 0 * 1 2 3 4 5 Baseline Placebo Galantamine 8 mg/day Galantamine 16 mg/day Galantamine 24 mg/day 1 2 3 Deterioration 4 5 Time (Weeks) *p<0. 05 vs. placebo; N=978 Tariot PN, Solomon PR, Morris JC, et al. Neurology. 2000(June 27); 54(12): 2269 -2276

Pharmacotherapy for Moderate to Severe Alzheimer’s Disease FDA Approved: ►Memantine Monotherapy l Combination Therapy l New Developments in Severe AD: ►Ch. EIs Monotherapy l Combination Therapy l

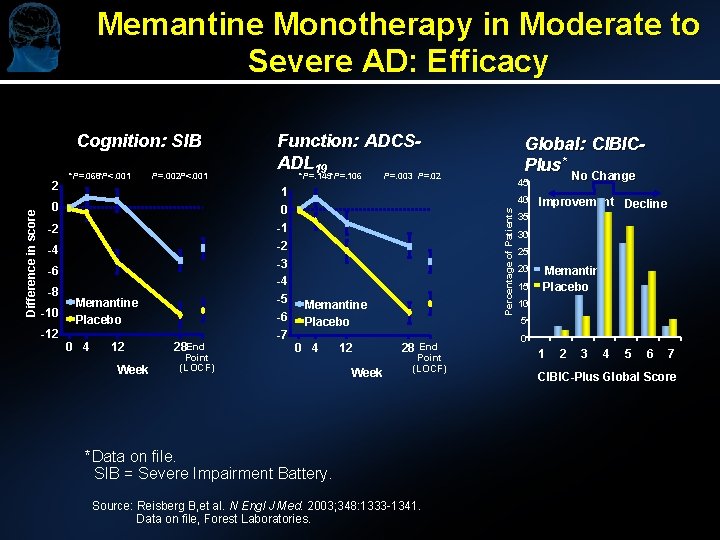
Memantine Monotherapy in Moderate to Severe AD: Efficacy Cognition: SIB *P=. 068*P<. 001 P=. 002 P<. 001 -2 -4 -6 -10 -12 P=. 003 P=. 02 1 0 -1 -2 -3 -4 0 -8 *P=. 145*P=. 106 -5 -6 Memantine Placebo 0 4 12 Week 28 End Point (LOCF) -7 Global: CIBICPlus* No Change 45 40 Percentage of Patients Difference in score 2 Function: ADCSADL 19 Memantine Placebo 0 4 12 Week 28 End Point (LOCF) *Data on file. SIB = Severe Impairment Battery. Source: Reisberg B, et al. N Engl J Med. 2003; 348: 1333 -1341. Data on file, Forest Laboratories. Improvement Decline 35 30 25 20 15 Memantine Placebo 10 5 0 1 2 3 4 5 6 7 CIBIC-Plus Global Score

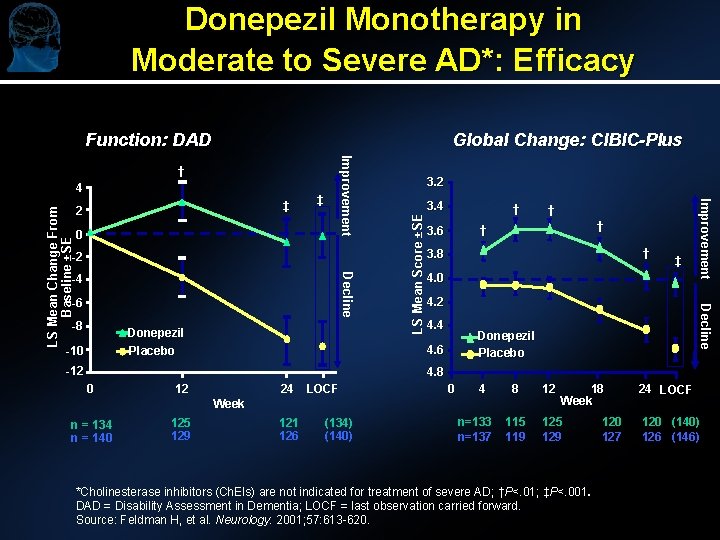
Donepezil Monotherapy in Moderate to Severe AD*: Efficacy Function: DAD Global Change: CIBIC-Plus ‡ 0 -2 Decline -4 -6 -8 Donepezil -10 LS Mean Score ±SE ‡ 2 3. 4 † -12 † † 3. 6 † 3. 8 ‡ 4. 0 4. 2 4. 4 Donepezil Placebo 4. 6 Placebo † Decline LS Mean Change From Baseline ±SE 4 Improvement † 4. 8 0 12 24 LOCF 0 4 8 12 115 119 125 129 Week n = 134 n = 140 125 129 121 126 (134) (140) n=133 n=137 18 Week *Cholinesterase inhibitors (Ch. EIs) are not indicated for treatment of severe AD; †P<. 01; ‡P<. 001. DAD = Disability Assessment in Dementia; LOCF = last observation carried forward. Source: Feldman H, et al. Neurology. 2001; 57: 613 -620. 120 127 24 LOCF 120 (140) 126 (146)

Increased Probability of Institutionalization by Disease Severity Probability of Institutionalization 1. 0 0. 867 0. 8 0. 6 0. 345 0. 4 0. 2 0. 017 0. 0 Mild (MMSE: 21 -30) Moderate (MMSE: 11 -20) Severe (MMSE: 0 -10) Severity of AD Hauber AB, Gnanasakthy, Snyder EH, et al. Pharmacoeconomics. 2000(April); 17(4): 351 -360

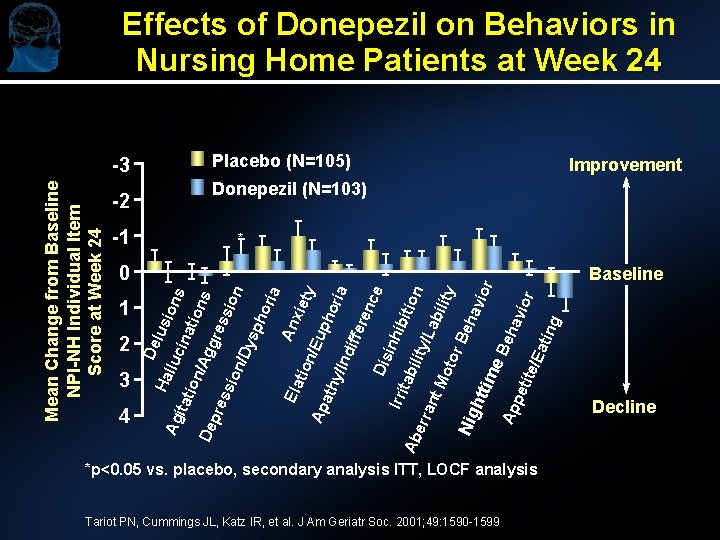
Effects of Donepezil on Behaviors in Nursing Home Patients at Week 24 Placebo (N=105) Improvement Donepezil (N=103) -2 -1 * r Baseline pet ite/ Ea tin g ior hav Be Ap Nig htt ime Be ha vio ty bili Mo tor /La ant Ab err Irri tab ility ibit ion e Dis inh enc ria ath y/In dif fer ho ty Ap Ela tio n/E up xie a An ori sph on De pre ssi on /Dy ssi ns gre ion /Ag ina tio ion lus itat 4 lluc 3 Ag 2 De 1 s 0 Ha Mean Change from Baseline NPI-NH Individual Item Score at Week 24 -3 *p<0. 05 vs. placebo, secondary analysis ITT, LOCF analysis Tariot PN, Cummings JL, Katz IR, et al. J Am Geriatr Soc. 2001; 49: 1590 -1599 Decline

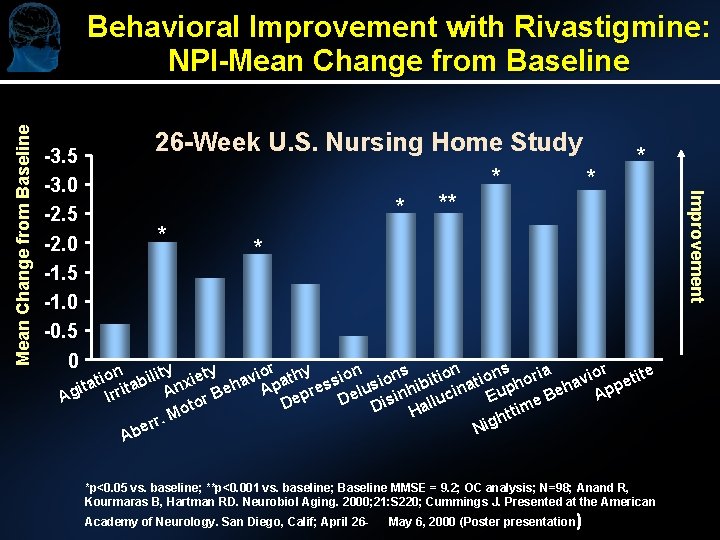
-3. 5 -3. 0 -2. 5 -2. 0 -1. 5 -1. 0 -0. 5 0 26 -Week U. S. Nursing Home Study * * * * * n lity xiety avior athy sion ions itions oria avior etite o i i t b ita rrita An r Beh Ap epres Delus inhib ucina Euph Beh App I Ag e is ll D to a D m o i t H t r. M gh r i e N Ab *p<0. 05 vs. baseline; **p<0. 001 vs. baseline; Baseline MMSE = 9. 2; OC analysis; N=98; Anand R, Kourmaras B, Hartman RD. Neurobiol Aging. 2000; 21: S 220; Cummings J. Presented at the American Academy of Neurology. San Diego, Calif; April 26 - May 6, 2000 (Poster presentation) Improvement Mean Change from Baseline Behavioral Improvement with Rivastigmine: NPI-Mean Change from Baseline

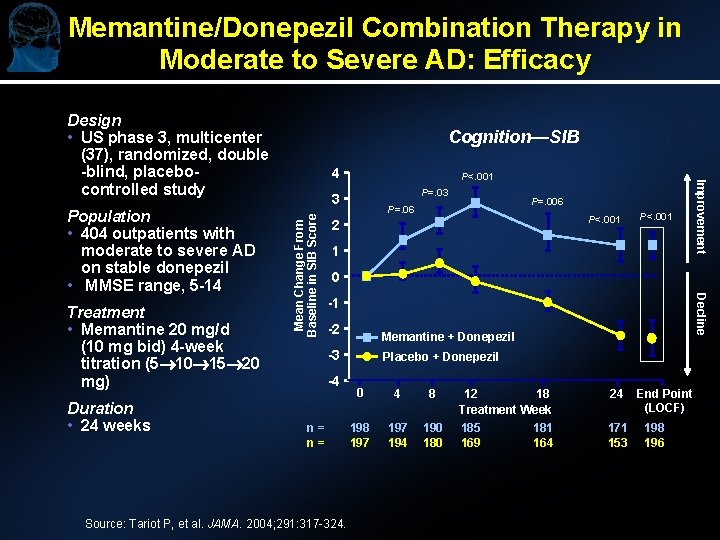
Memantine/Donepezil Combination Therapy in Moderate to Severe AD: Efficacy Design • US phase 3, multicenter (37), randomized, double -blind, placebocontrolled study Duration • 24 weeks P<. 001 P=. 03 Mean Change From Baseline in SIB Score 3 P=. 006 P=. 06 P<. 001 2 P<. 001 1 0 Decline Treatment • Memantine 20 mg/d (10 mg bid) 4 -week titration (5 10 15 20 mg) 4 Improvement Population • 404 outpatients with moderate to severe AD on stable donepezil • MMSE range, 5 -14 Cognition—SIB -1 -2 Memantine + Donepezil -3 -4 n= n= Source: Tariot P, et al. JAMA. 2004; 291: 317 -324. Placebo + Donepezil 0 4 8 197 194 190 180 12 18 Treatment Week 185 181 169 164 24 171 153 End Point (LOCF) 198 196

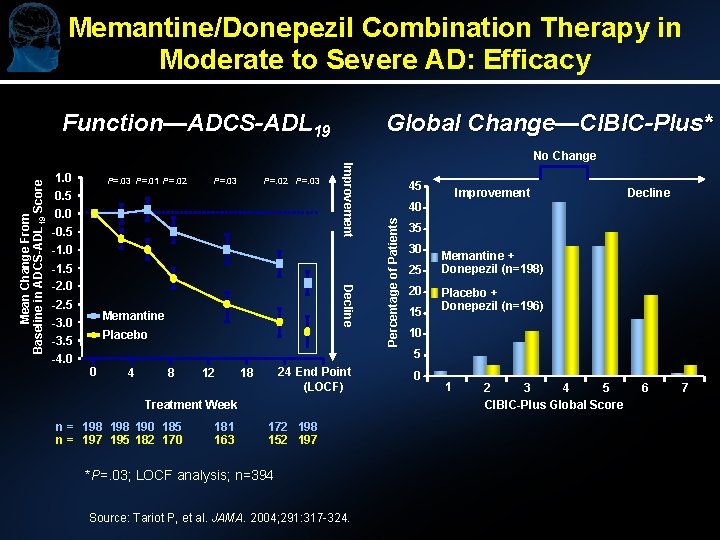
Memantine/Donepezil Combination Therapy in Moderate to Severe AD: Efficacy Function—ADCS-ADL 19 Global Change—CIBIC-Plus* P=. 03 P=. 01 P=. 02 P=. 03 0. 5 0. 0 -0. 5 -1. 0 -2. 5 -3. 0 -3. 5 -4. 0 Decline -1. 5 -2. 0 Memantine Placebo 45 Improvement Decline 40 Percentage of Patients 1. 0 Improvement Mean Change From Baseline in ADCS-ADL 19 Score No Change 35 30 25 20 15 Memantine + Donepezil (n=198) Placebo + Donepezil (n=196) 10 5 0 4 8 12 24 End Point (LOCF) 18 Treatment Week n = 198 190 185 n = 197 195 182 170 181 163 172 198 152 197 *P=. 03; LOCF analysis; n=394 Source: Tariot P, et al. JAMA. 2004; 291: 317 -324. 0 1 2 3 4 5 CIBIC-Plus Global Score 6 7

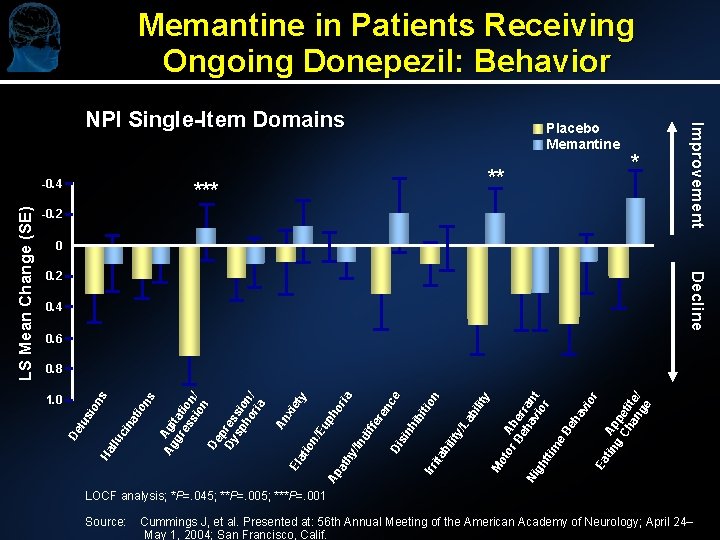
Memantine in Patients Receiving Ongoing Donepezil: Behavior ** * -0. 2 0 0. 2 Decline 0. 4 0. 6 1. 0 An xi El et at y io n/ Eu ph Ap or at ia hy /In di ffe re nc Di e si nh ib iti Irr on ita bi lit y/ La bi lit y M ot or Ab Be er ha ran Ni vi t gh or tti m e Be ha vi or Ea tin Ap g pe Ch ti an te/ ge 0. 8 De lu sio ns Ha llu ci na tio ns Ag Agi gr tat es ion si / on De p Dy res sp sio ho n/ ria LS Mean Change (SE) -0. 4 Placebo Memantine Improvement NPI Single-Item Domains LOCF analysis; *P=. 045; **P=. 005; ***P=. 001 Source: Cummings J, et al. Presented at: 56 th Annual Meeting of the American Academy of Neurology; April 24– May 1, 2004; San Francisco, Calif.

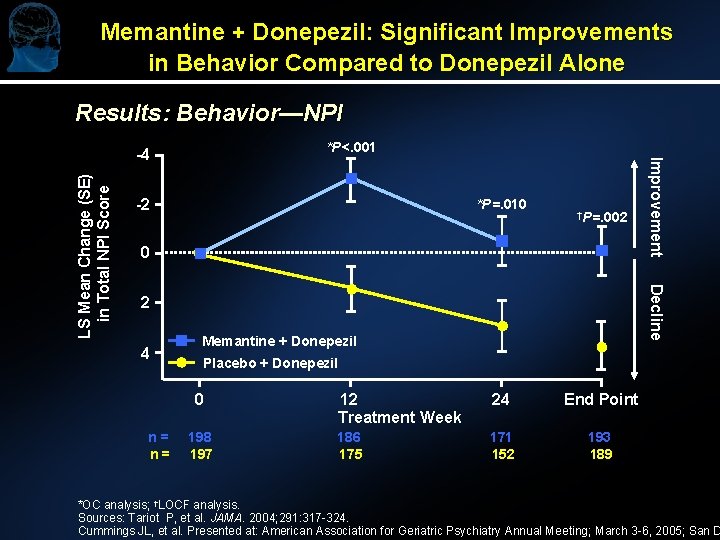
Memantine + Donepezil: Significant Improvements in Behavior Compared to Donepezil Alone Results: Behavior—NPI *P<. 001 *P=. 010 †P=. 002 0 Decline LS Mean Change (SE) in Total NPI Score -2 2 4 Memantine + Donepezil Placebo + Donepezil 0 n= n= Improvement -4 198 197 12 Treatment Week 24 End Point 186 175 171 152 193 189 *OC analysis; †LOCF analysis. Sources: Tariot P, et al. JAMA. 2004; 291: 317 -324. Cummings JL, et al. Presented at: American Association for Geriatric Psychiatry Annual Meeting; March 3 -6, 2005; San D

Tolerability of Memantine/Donepezil Combination Therapy Adverse Event* Placebo + Donepezil (n=201) n (%) Memantine + Donepezil (n=202) n (%) 24 (11. 9) 19 (9. 4) 4 (2. 0) 16 (7. 9) Fall 14 (7. 0) 15 (7. 4) Influenza-like symptoms 13 (6. 5) 15 (7. 4) Dizziness 16 (8. 0) 14 (6. 9) Headache 5 (2. 5) 13 (6. 4) Urinary tract infection 10 (5. 0) 12 (5. 9) Urinary incontinence 6 (3. 0) 11 (5. 4) Accidental injury 16 (8. 0) 10 (5. 0) Upper respiratory tract infection 13 (6. 5) 10 (5. 0) 8 (4. 0) 10 (5. 0) Diarrhea 17 (8. 5) 9 (4. 5) Fecal incontinence 10 (5. 0) 4 (2. 0) Agitation Confusion Peripheral edema *Adverse events reported in 5% of either treatment group. Source: Tariot P, et al. JAMA. 2004; 291: 317 -324.

Evaluating Response ► Assess: l At baseline l After reaching maximal tolerated dose l Every 6 – 12 months l If change in status ► Family/Patient/Nursing interview ► Documentation in medical record l Cooperation in activities, tolerance of groups l ADL abilities & tolerance with assistance Eating, toileting, dressing, showering, etc. l Routine and psychotropic medication usage

Summary ► AD is an expensive illness in human and economic terms for patients, their caregivers, and society. ► Diagnosis is often not made, especially in early and mild AD; clinical nihilism can interfere with initiating or sustaining treatment. The long term setting brings additional clinical challenges. ► Cholinesterase inhibitors and NMDA receptor antagonists attenuate symptomatic decline and may modify disease progression. ► Early treatment pays off; delaying treatment has long-term consequences.

Summary ► Ch. Els (mild to moderate AD) and memantine (moderate to severe AD) monotherapies are associated with less decline (vs placebo) in cognition and function ► Although not indicated, newer data support a role for memantine in mild AD and Ch. EIs in severe AD ► In moderate to severe AD, patients treated with combination therapy (ie, memantine + Ch. EIs) exhibited improved cognitive outcomes and delayed functional decline (vs patients treated with Ch. EI only)
 Which is an alternative of log based recovery
Which is an alternative of log based recovery D orbital shape
D orbital shape Absorption spectrum
Absorption spectrum Georgia alzheimers planning
Georgia alzheimers planning Fast scale for dementia
Fast scale for dementia Alzheimers sjukdom
Alzheimers sjukdom Alzheimers nz conference 2020
Alzheimers nz conference 2020 Historical research
Historical research Alzheimers society contented dementia
Alzheimers society contented dementia Alzheimer's eye test joke
Alzheimer's eye test joke Natural history and spectrum of disease
Natural history and spectrum of disease Spectrum of disease
Spectrum of disease Dyesthesiasis
Dyesthesiasis Shahzad ahmad md
Shahzad ahmad md Maple syrup urine disease treatment
Maple syrup urine disease treatment Modern treatment of heart disease
Modern treatment of heart disease Disease control phase
Disease control phase Bharathi viswanathan
Bharathi viswanathan Full spectrum command
Full spectrum command Zechariah
Zechariah Zechariah 4:8
Zechariah 4:8 University community plan
University community plan Temporary update problem in dbms
Temporary update problem in dbms Sab update.com 2021
Sab update.com 2021 Routing area update
Routing area update Update data sisdmk
Update data sisdmk Gtcs prd examples
Gtcs prd examples Position update formula
Position update formula Position update formula
Position update formula Fiberhome, firmware
Fiberhome, firmware Move update compliance
Move update compliance Mdh situation update
Mdh situation update Iqcs responder update sheet
Iqcs responder update sheet Utils ctl update ctlfile
Utils ctl update ctlfile 811 ticket number
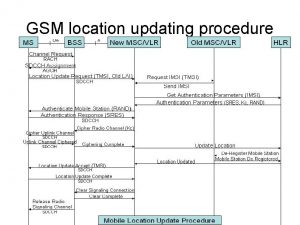
811 ticket number Location update procedure
Location update procedure Chrome update
Chrome update Dhl ocean freight market update
Dhl ocean freight market update Ddns-update-style none
Ddns-update-style none Food product knowledge training
Food product knowledge training Gov.ukdbs
Gov.ukdbs Non-updating function module called for updating
Non-updating function module called for updating Unity connection to the update server failed
Unity connection to the update server failed