Clinical Trials gov Identifier NCT 00390832 Prospective Randomized



![REVIVAL-3 Blood Count Reticulocytes * reticulocytes [%] 40 Epo 35 * 18 * * REVIVAL-3 Blood Count Reticulocytes * reticulocytes [%] 40 Epo 35 * 18 * *](https://slidetodoc.com/presentation_image_h2/d0b0eeb2b93f53a8f971badb26126508/image-16.jpg)
![REVIVAL-3 Primary End Point EF in MRI [%] P =. 91 Epo Placebo n=52 REVIVAL-3 Primary End Point EF in MRI [%] P =. 91 Epo Placebo n=52](https://slidetodoc.com/presentation_image_h2/d0b0eeb2b93f53a8f971badb26126508/image-17.jpg)
![REVIVAL-3 Angiographic Results Increase in EF over 6 months Delta [%] P =. 38 REVIVAL-3 Angiographic Results Increase in EF over 6 months Delta [%] P =. 38](https://slidetodoc.com/presentation_image_h2/d0b0eeb2b93f53a8f971badb26126508/image-20.jpg)

- Slides: 24

Clinical. Trials. gov Identifier: NCT 00390832 Prospective, Randomized, Double-Blind, Placebocontrolled Trial of Erythropoietin in Patients With STSegment Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention (REVIVAL-3) Ilka Ott, Stefanie Schulz, Julinda Mehilli, Stephanie Fichtner, Martin Hadamitzky, Tarek Ibrahim, Janina Hackl, Katharina Hoppe, Stefan Martinoff, Josef Dirschinger, Adnan Kastrati, Albert Schömig Deutsches Herzzentrum und 1. Medizinische Klinik der Technischen Universität München No conflict of interest to disclose

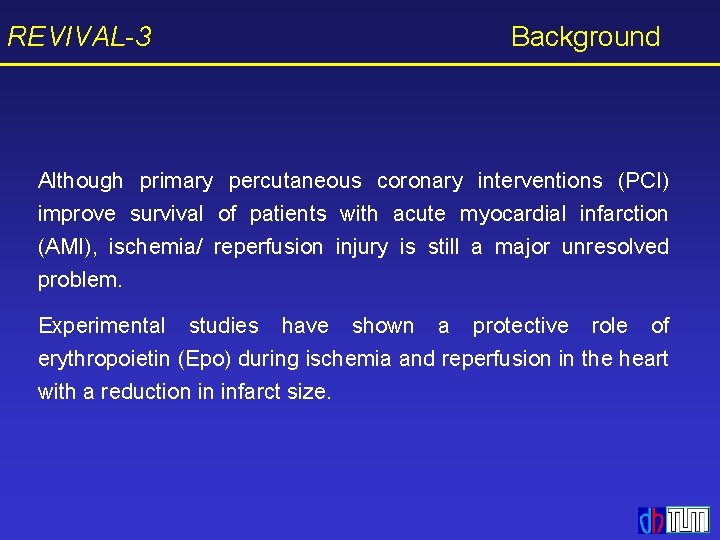
REVIVAL-3 Background Although primary percutaneous coronary interventions (PCI) improve survival of patients with acute myocardial infarction (AMI), ischemia/ reperfusion injury is still a major unresolved problem. Experimental studies have shown a protective role of erythropoietin (Epo) during ischemia and reperfusion in the heart with a reduction in infarct size.

REVIVAL-3 Erythropoietin (Epo): Experimental Studies control Epo Epo reduces infarct size and improves myocardial function. This was associated with a decrease in apoptotic cells, an increase in EPC recruitment and neovascularization. Parsa et al. 2003; Westenbrink et al. 2007

REVIVAL-3 Erythropoietin (EPO): Clinical Studies In patients with ischemic stroke high-dose Epoetin beta improved clinical outcome and reduced infarct size by trend (n=40) Ehrenreich et al. 2002; Mol Med In patients with AMI high-dose Darbopoietin was safe and well tolerated (n=22) Lipsic et al. 2006 Cardiovascular Drugs and Therapy

REVIVAL-3 Objective. . . was to assess the effect of high-dose erythropoietin in patients with acute ST-elevation myocardial infarction treated with primary PCI

REVIVAL-3 Inclusion Criteria Patients with acute ST-elevation myocardial infarction with primary PCI • chest pain lasting more than 20 min • ≥ 0. 1 m. V of ST-segment elevation in ≥ 2 limb leads or ≥ 0. 2 m. V in ≥ 2 contiguous precordial leads or new left bundle branch block on surface ECG Angiographically left ventricular ejection fraction <50% Written, informed consent

REVIVAL-3 Key Exclusion Criteria Ø Age > 80 or < 18 years Ø Cardiogenic shock or prolonged cardiopulmonary resuscitation Ø Previous MI Ø Severe uncontrolled hypertension (>180 mm. Hg, unresponsive to therapy) Ø Hematological disorders such as essential thrombocytosis, megakaryoblastic leukemia, polycythemia vera Ø Relevant hematologic deviations (hemoglobin < 10. 0 g/L or > 15. 5 g/L, platelet count < 100 x 109/L or > 600 x 109/L) Ø Coronary intervention within the last 30 days Ø Any contraindication to magnetic resonance imaging Ø Known allergy to study medication, pregnancy, prior inclusion in the study

Study Therapy REVIVAL-3 1. balloon inflation 24 hrs 48 hrs 3. 3 x 104 U Epoetin beta Placebo n=68 138 patients with STEMI Cath Lab Randomization n=70 1. balloon inflation 24 hrs 48 hrs

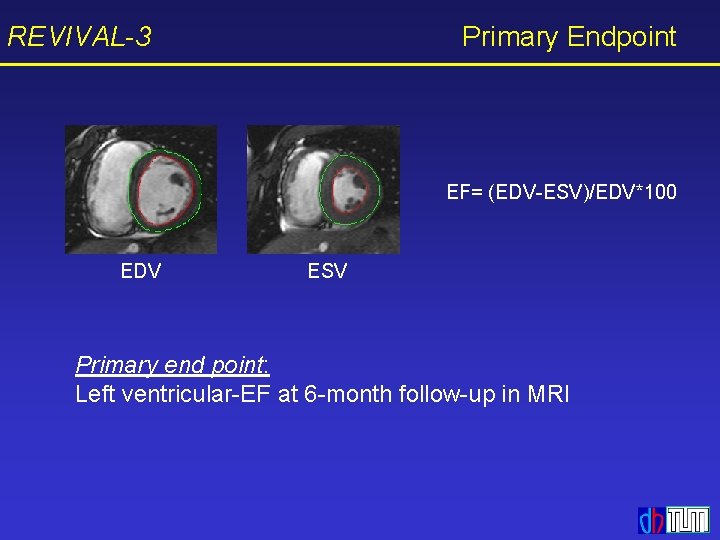
REVIVAL-3 Primary Endpoint EF= (EDV-ESV)/EDV*100 EDV ESV Primary end point: Left ventricular-EF at 6 -month follow-up in MRI

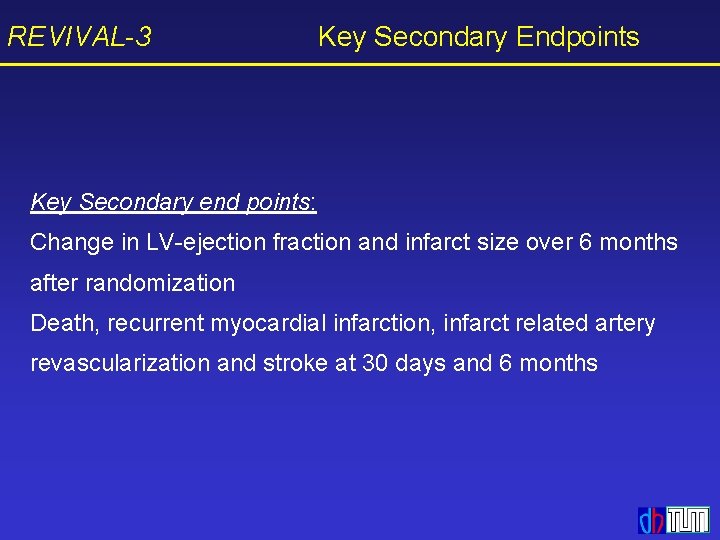
REVIVAL-3 Key Secondary Endpoints Key Secondary end points: Change in LV-ejection fraction and infarct size over 6 months after randomization Death, recurrent myocardial infarction, infarct related artery revascularization and stroke at 30 days and 6 months

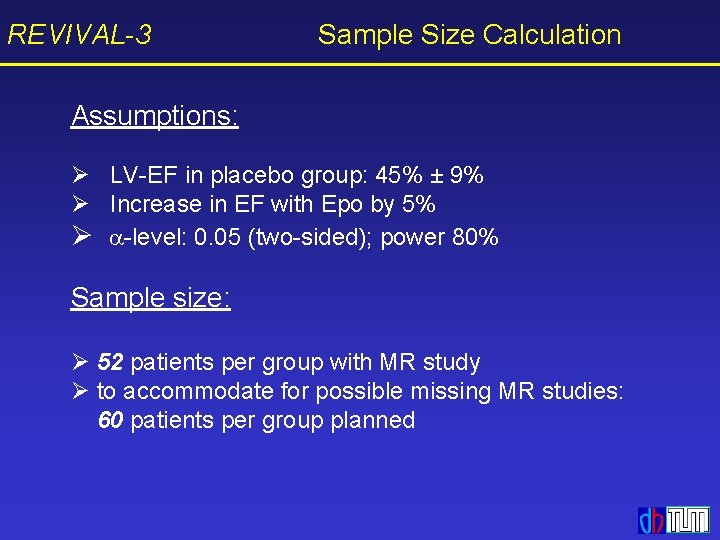
REVIVAL-3 Sample Size Calculation Assumptions: Ø LV-EF in placebo group: 45% ± 9% Ø Increase in EF with Epo by 5% Ø -level: 0. 05 (two-sided); power 80% Sample size: Ø 52 patients per group with MR study Ø to accommodate for possible missing MR studies: 60 patients per group planned

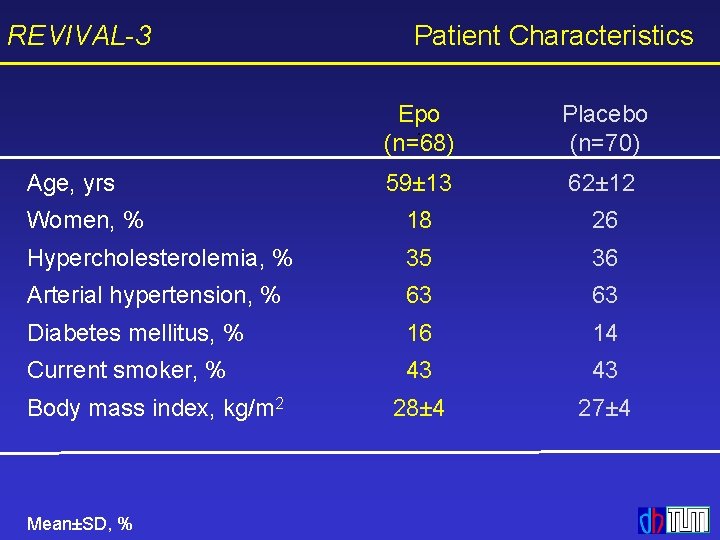
REVIVAL-3 Patient Characteristics Epo (n=68) Placebo (n=70) 59± 13 62± 12 Women, % 18 26 Hypercholesterolemia, % 35 36 Arterial hypertension, % 63 63 Diabetes mellitus, % 16 14 Current smoker, % 43 43 28± 4 27± 4 Age, yrs Body mass index, kg/m 2 Mean±SD, %

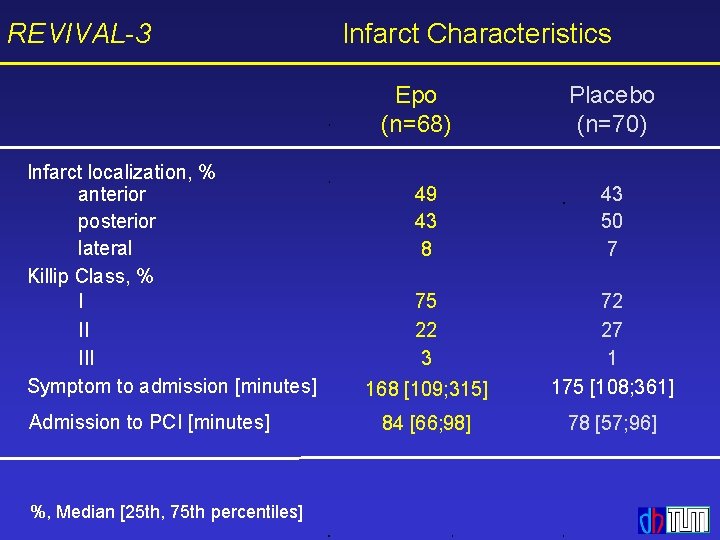
REVIVAL-3 Infarct Characteristics Epo (n=68) Infarct localization, % anterior posterior lateral Killip Class, % I II III Symptom to admission [minutes] Admission to PCI [minutes] %, Median [25 th, 75 th percentiles] Placebo (n=70) 49 43 8 43 50 7 75 22 3 168 [109; 315] 72 27 1 175 [108; 361] 84 [66; 98] 78 [57; 96]

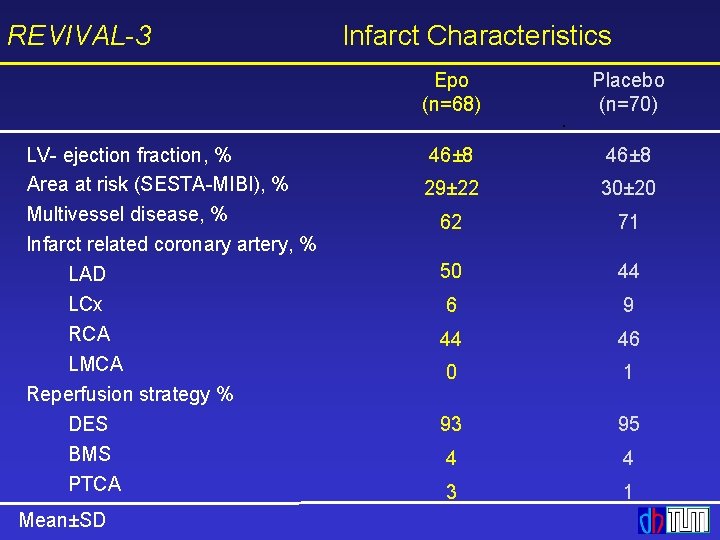
REVIVAL-3 LV- ejection fraction, % Area at risk (SESTA-MIBI), % Multivessel disease, % Infarct related coronary artery, % LAD LCx RCA LMCA Reperfusion strategy % DES BMS PTCA Mean±SD Infarct Characteristics Epo (n=68) Placebo (n=70) 46± 8 29± 22 30± 20 62 71 50 44 6 9 44 46 0 1 93 95 4 4 3 1

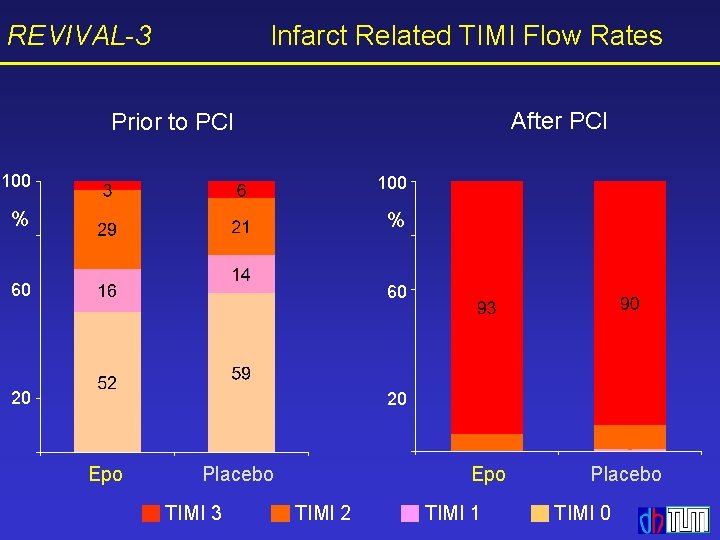
REVIVAL-3 Infarct Related TIMI Flow Rates After PCI Prior to PCI 100 % % 60 60 20 20 Epo Placebo TIMI 3 Epo TIMI 2 TIMI 1 Placebo TIMI 0
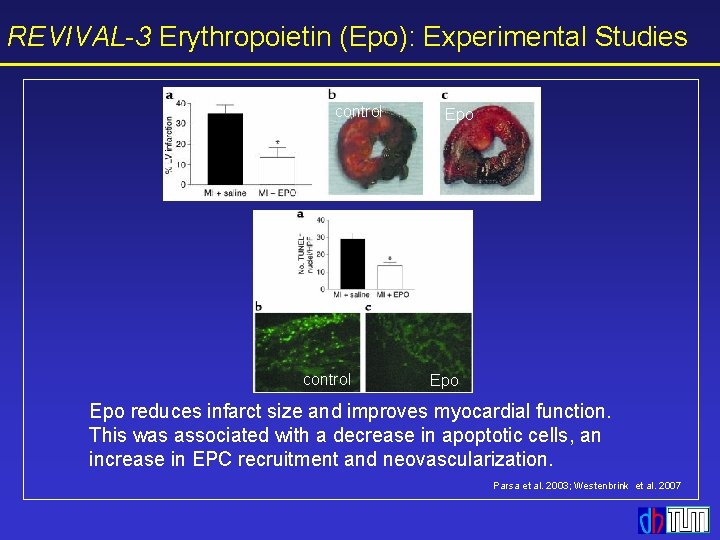
![REVIVAL3 Blood Count Reticulocytes reticulocytes 40 Epo 35 18 REVIVAL-3 Blood Count Reticulocytes * reticulocytes [%] 40 Epo 35 * 18 * *](https://slidetodoc.com/presentation_image_h2/d0b0eeb2b93f53a8f971badb26126508/image-16.jpg)
REVIVAL-3 Blood Count Reticulocytes * reticulocytes [%] 40 Epo 35 * 18 * * 30 * 25 Placebo 20 15 10 hemoglobin [mg/dl] 45 Hemoglobin 14 12 8 6 4 2 0 0 1 2 3 4 5 6 7 days after randomization Mean±SD Placebo 10 5 0 Epo 16 0 1 2 3 4 5 6 7 days after randomization * p < 0. 05
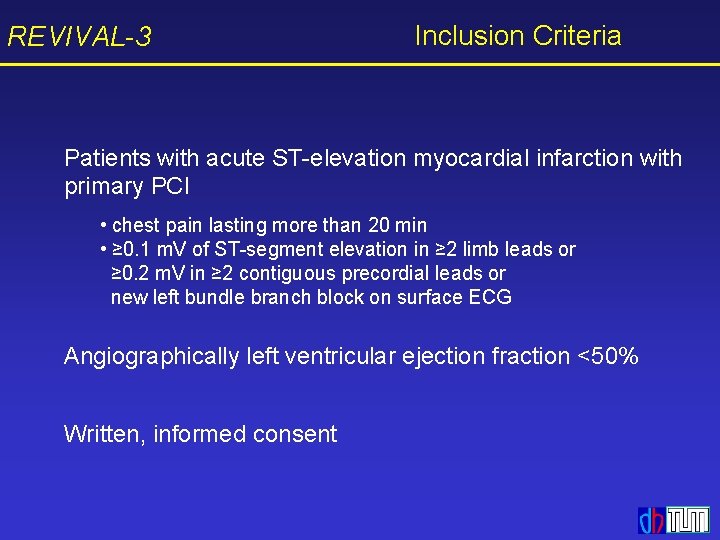
![REVIVAL3 Primary End Point EF in MRI P 91 Epo Placebo n52 REVIVAL-3 Primary End Point EF in MRI [%] P =. 91 Epo Placebo n=52](https://slidetodoc.com/presentation_image_h2/d0b0eeb2b93f53a8f971badb26126508/image-17.jpg)
REVIVAL-3 Primary End Point EF in MRI [%] P =. 91 Epo Placebo n=52 n=55 6 -month follow-up

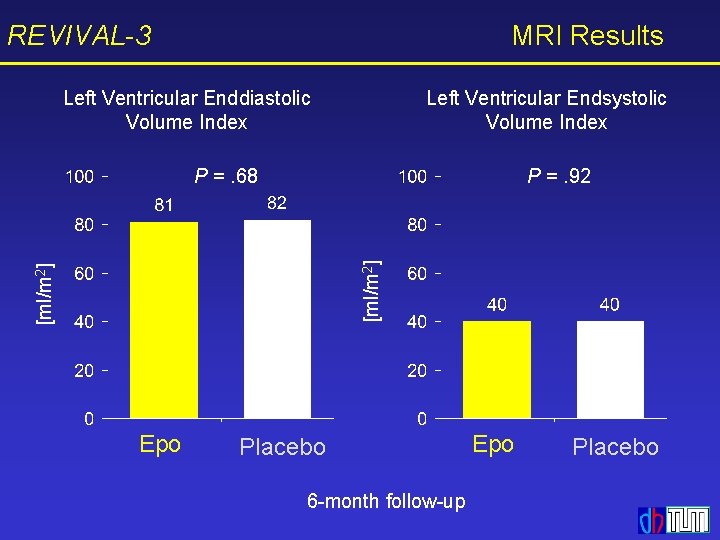
REVIVAL-3 MRI Results Left Ventricular Enddiastolic Volume Index Left Ventricular Endsystolic Volume Index P =. 92 [ml/m 2] P =. 68 Epo Placebo 6 -month follow-up Epo Placebo

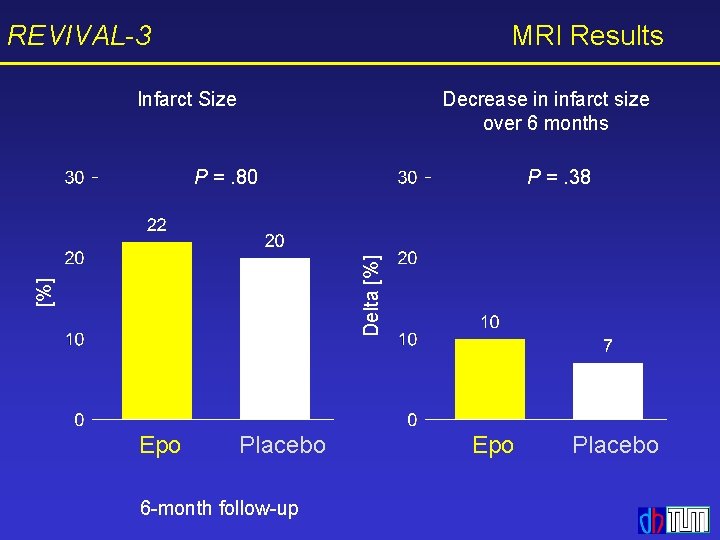
REVIVAL-3 MRI Results Infarct Size Decrease in infarct size over 6 months P =. 38 [%] Delta [%] P =. 80 Epo Placebo 6 -month follow-up Epo Placebo
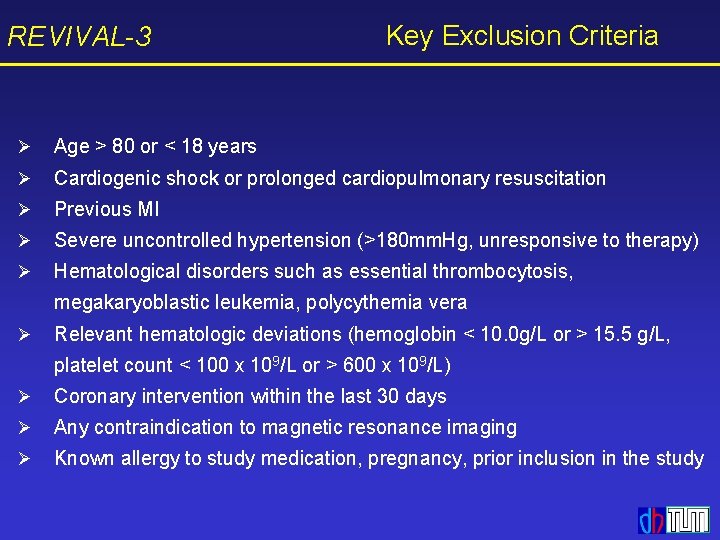
![REVIVAL3 Angiographic Results Increase in EF over 6 months Delta P 38 REVIVAL-3 Angiographic Results Increase in EF over 6 months Delta [%] P =. 38](https://slidetodoc.com/presentation_image_h2/d0b0eeb2b93f53a8f971badb26126508/image-20.jpg)
REVIVAL-3 Angiographic Results Increase in EF over 6 months Delta [%] P =. 38 Epo Placebo 6 -month follow-up

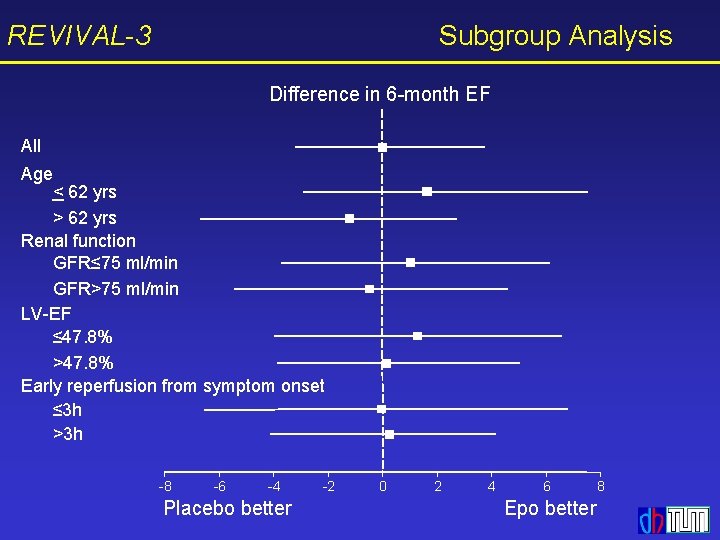
REVIVAL-3 Subgroup Analysis Difference in 6 -month EF All Age < 62 yrs > 62 yrs Renal function GFR≤ 75 ml/min GFR>75 ml/min LV-EF ≤ 47. 8% >47. 8% Early reperfusion from symptom onset ≤ 3 h >3 h -8 -6 -4 Placebo better -2 0 2 4 6 Epo better 8

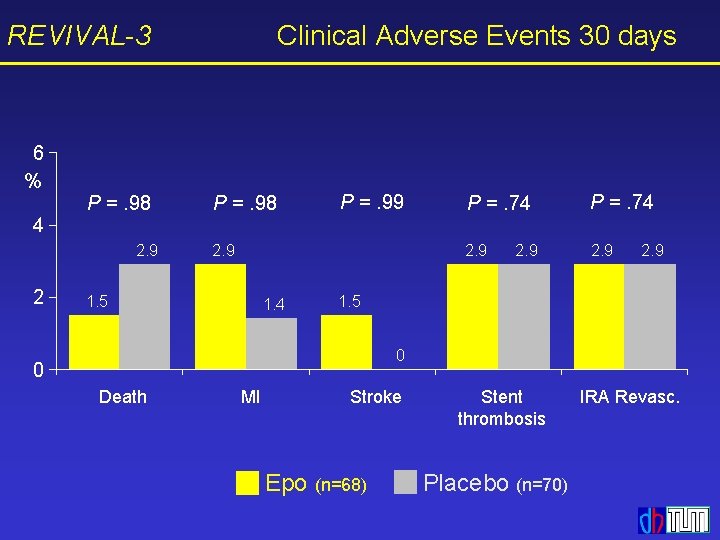
REVIVAL-3 Clinical Adverse Events 30 days 6 % 4 P =. 98 2. 9 2 P =. 98 P =. 99 2. 9 1. 5 1. 4 P =. 74 2. 9 1. 5 0 0 Death MI Stroke Epo (n=68) Stent thrombosis Placebo (n=70) IRA Revasc.

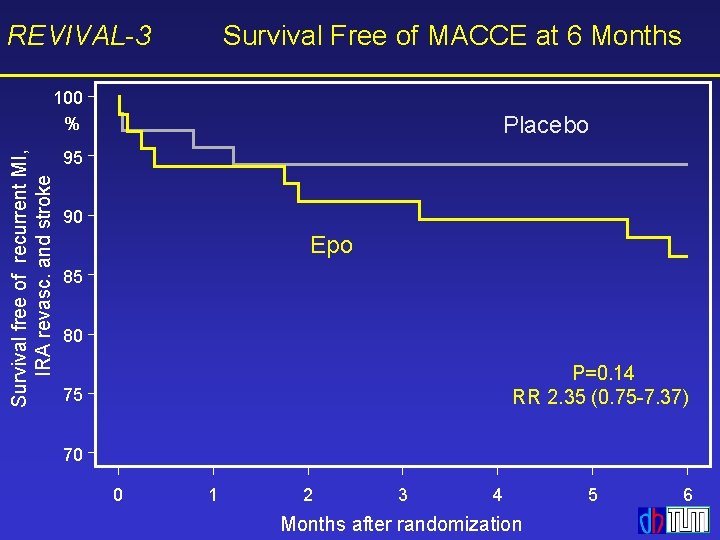
REVIVAL-3 Survival Free of MACCE at 6 Months Survival free of recurrent MI, IRA revasc. and stroke 100 % Placebo 95 90 Epo 85 80 P=0. 14 RR 2. 35 (0. 75 -7. 37) 75 70 0 1 2 3 4 Months after randomization 5 6

REVIVAL-3 Conclusion High dose epoetin beta does not improve left ventricular function or reduce infarct size in patients with ST-elevation myocardial infarction treated with primary PCI. The trend towards a higher risk of adverse clinical events should be taken into account before planing future investigations with this drug in patients with AMI.