Clinical Trials Flow Process The life Cycle of





























- Slides: 47

Clinical Trials Flow Process: The life Cycle of Clinical Trials Tamer Hifnawy MD. Dr. PH Associate Professor Public Health & Community Medicine Faculty of Medicine – BSU- Egypt College of Dentistry Taibah University- KSA Vice Dean For Quality, Development & International Affairs Certified Trainer for International Research Ethics

Objectives p p Developing/Writing a protocol. Developing an Investigator Site File (ISF) – Regulatory Binder. Screening, Recruitment, Enrollment and Retention. . End of Study Visit.

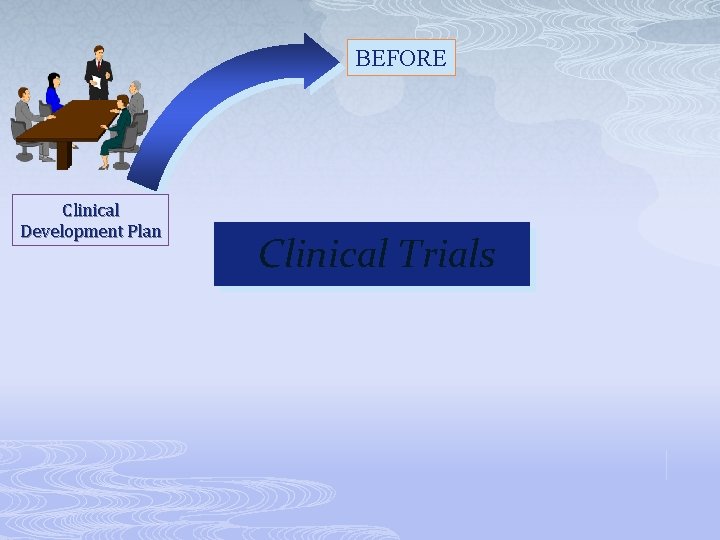
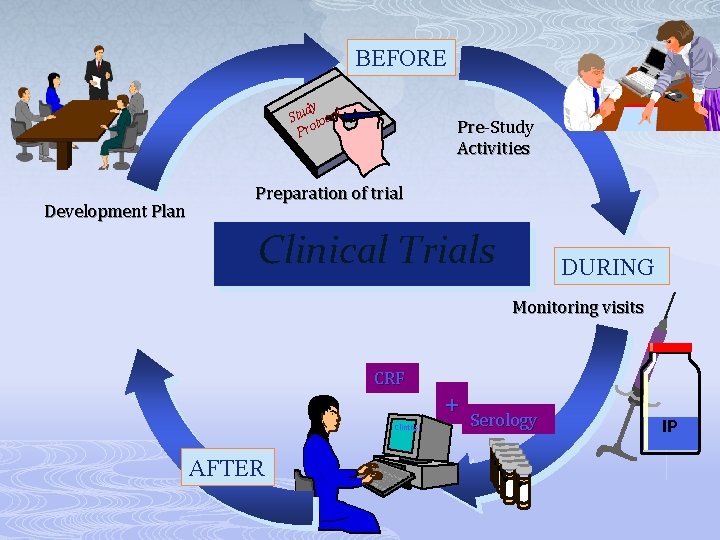
BEFORE Clinical Development Plan Clinical Trials

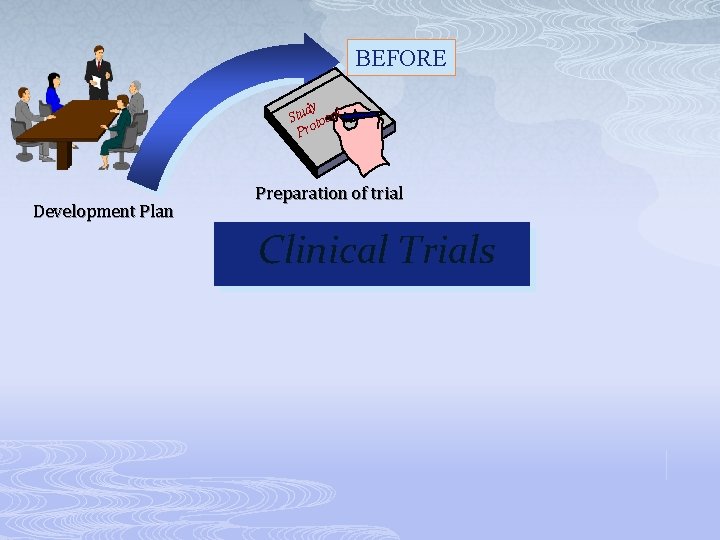
BEFORE dy Stu tocol Pro Development Plan Preparation of trial Clinical Trials

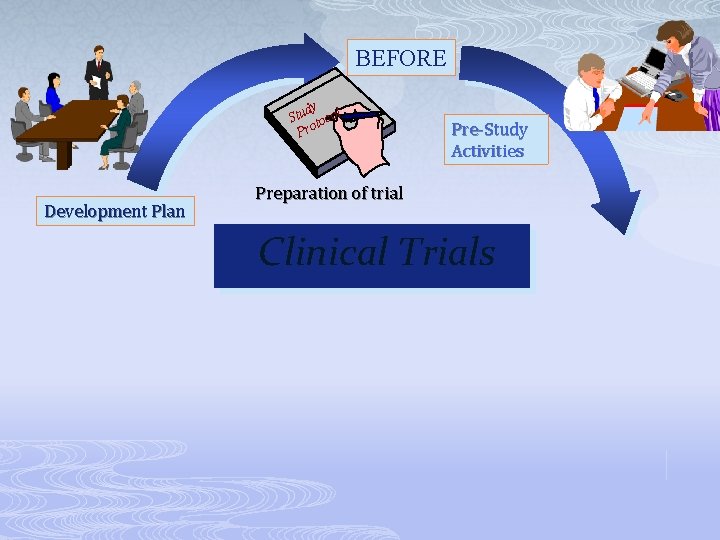
BEFORE dy Stu tocol Pro Development Plan Pre-Study Activities Preparation of trial Clinical Trials

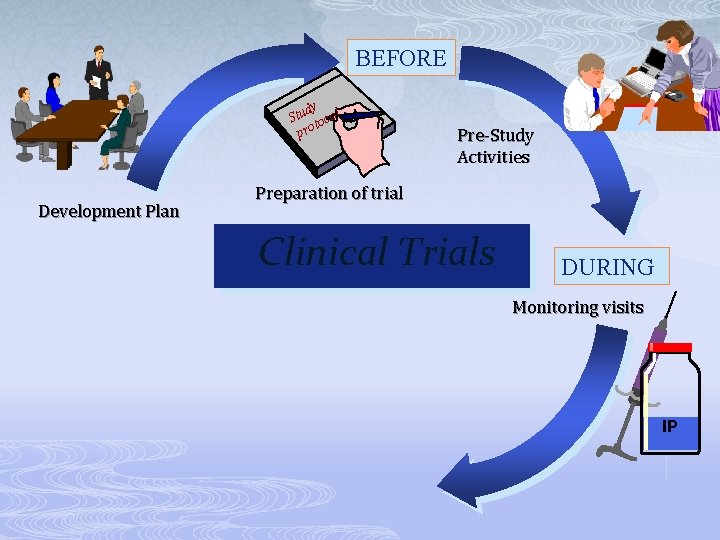
BEFORE dy Stu tocol pro Development Plan Pre-Study Activities Preparation of trial Clinical Trials DURING Monitoring visits IP

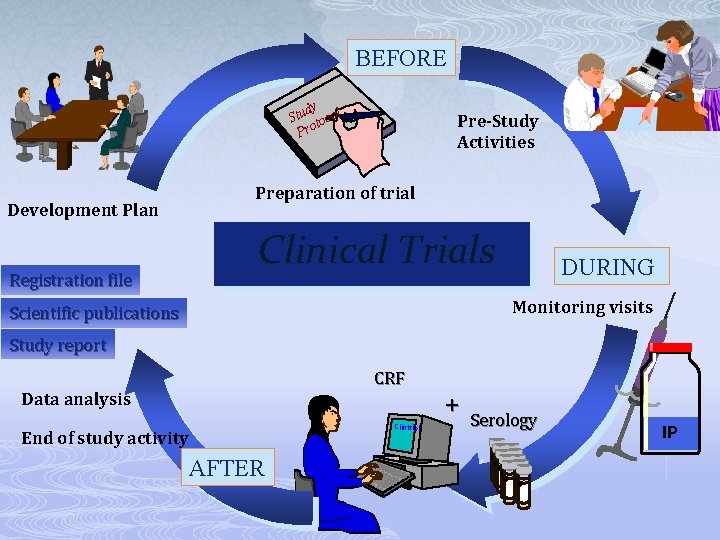
BEFORE dy Stu tocol Pro Development Plan Pre-Study Activities Preparation of trial Clinical Trials DURING Monitoring visits CRF Clintrial AFTER + Serology IP

BEFORE dy Stu tocol Pro Development Plan Registration file Pre-Study Activities Preparation of trial Clinical Trials DURING Monitoring visits Scientific publications Study report CRF Data analysis Clintrial End of study activity AFTER + Serology IP

Designing a protocol

General Information p p p Protocol title, and date. Name and address of the Investigator & sponsor Name, title, address, and telephone number(s) of the sponsor's medical expert for the trial.

Background Information p p Name and description of the investigational product(s). A summary of findings from nonclinical studies that potentially have clinical significance and from clinical trials that are relevant to the trial. Summary of the known and potential risks and benefits, if any, to human subjects. Description of and justification for the route of administration, dosage regimen, and treatment period(s).

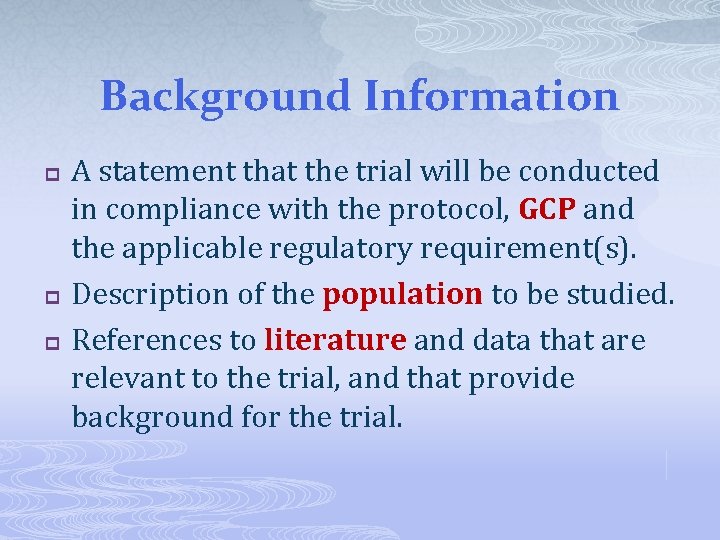
Background Information p p p A statement that the trial will be conducted in compliance with the protocol, GCP and the applicable regulatory requirement(s). Description of the population to be studied. References to literature and data that are relevant to the trial, and that provide background for the trial.

Trial Objectives and Purpose p A detailed description of the objectives and the purpose of the trial.

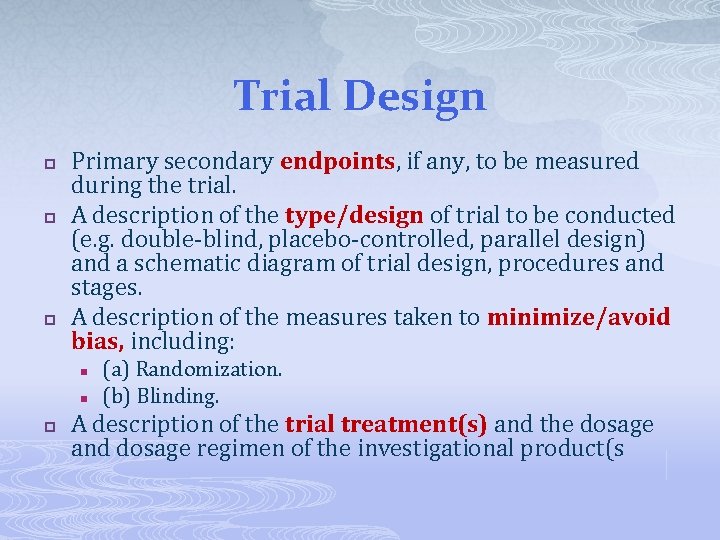
Trial Design p p p Primary secondary endpoints, if any, to be measured during the trial. A description of the type/design of trial to be conducted (e. g. double-blind, placebo-controlled, parallel design) and a schematic diagram of trial design, procedures and stages. A description of the measures taken to minimize/avoid bias, including: n n p (a) Randomization. (b) Blinding. A description of the trial treatment(s) and the dosage and dosage regimen of the investigational product(s

Selection and Withdrawal of Subjects p p p Subject inclusion criteria. Subject exclusion criteria. Subject withdrawal criteria (i. e. terminating investigational product treatment/trial treatment) and procedures.

Assessment of Efficacy p p Specification of the efficacy parameters. Methods and timing for assessing, recording, and analysing of efficacy parameters.

Assessment of Safety p p p Specification , methods & timing of safety parameters. Procedures for eliciting reports for recording and reporting adverse event. The type and duration of the follow-up of subjects after adverse events.

Statistics p p Statistical methods to be employed, and planned interim analysis(ses). Sample size & its justification (Power). The level of significance to be used. Criteria for the termination of the trial.

Quality Control and Quality Assurance Ethics Data Handling and Record Keeping Financing and Insurance

Objectives p p p Developing/Writing a protocol. Developing an Investigator Site File (ISF) – Regulatory Binder. Screening, Recruitment, Enrollment and Retention.

Investigator Study File & Essential Documents

Definition Essential Documents: Documents which individually and collectively permit evaluation of the conduct of a study and the quality of the data produced

Sections p Grouped in 3 sections: 1) before the clinical phase of the trial commences, 2) during the clinical conduct of the trial, and 3) after completion or termination of the trial

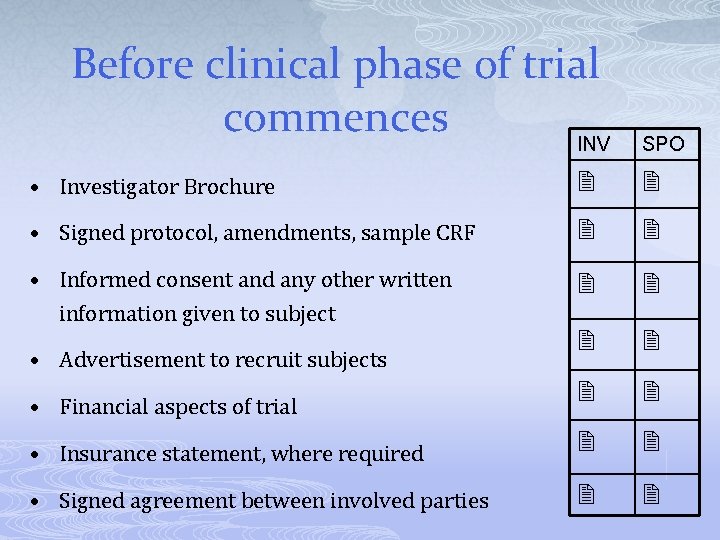
Before clinical phase of trial commences INV SPO • Investigator Brochure • Signed protocol, amendments, sample CRF • Informed consent and any other written information given to subject • Insurance statement, where required • Signed agreement between involved parties • Advertisement to recruit subjects • Financial aspects of trial

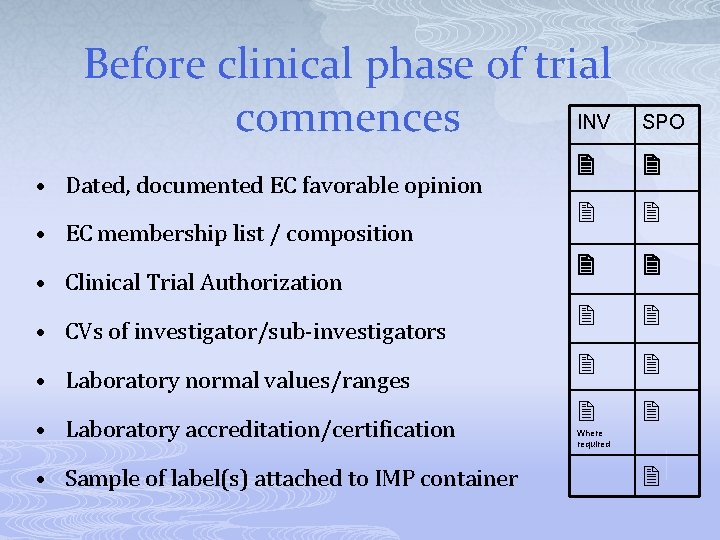
Before clinical phase of trial INV commences • Dated, documented EC favorable opinion • EC membership list / composition • Clinical Trial Authorization • CVs of investigator/sub-investigators • Laboratory normal values/ranges • Laboratory accreditation/certification • Sample of label(s) attached to IMP container SPO Where required

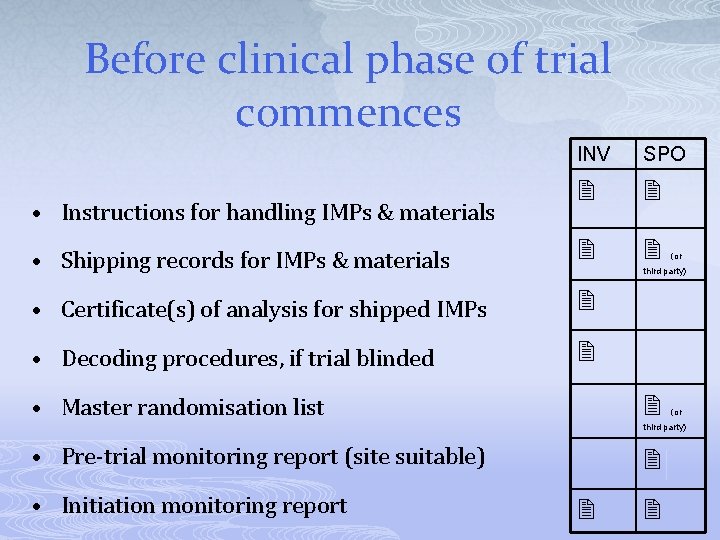
Before clinical phase of trial commences INV SPO • Shipping records for IMPs & materials • Certificate(s) of analysis for shipped IMPs • Decoding procedures, if trial blinded • Instructions for handling IMPs & materials (or third party) • Master randomisation list (or third party) • Pre-trial monitoring report (site suitable) • Initiation monitoring report

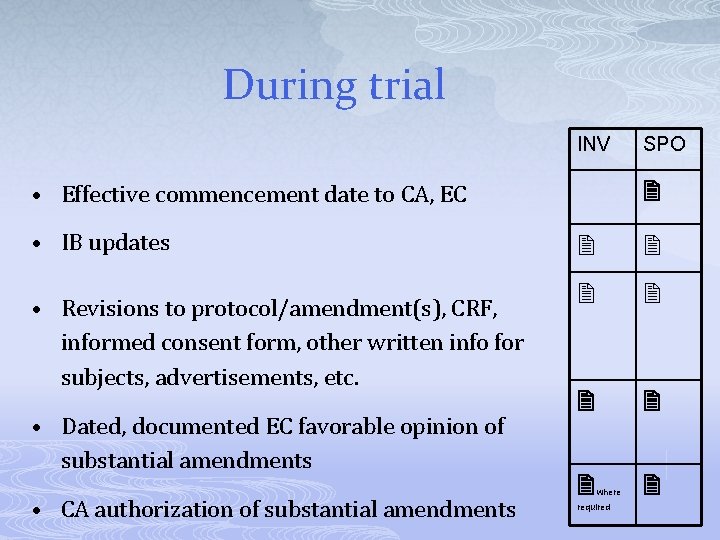
During trial INV • Effective commencement date to CA, EC • IB updates • Revisions to protocol/amendment(s), CRF, informed consent form, other written info for subjects, advertisements, etc. • Dated, documented EC favorable opinion of substantial amendments • CA authorization of substantial amendments SPO where required

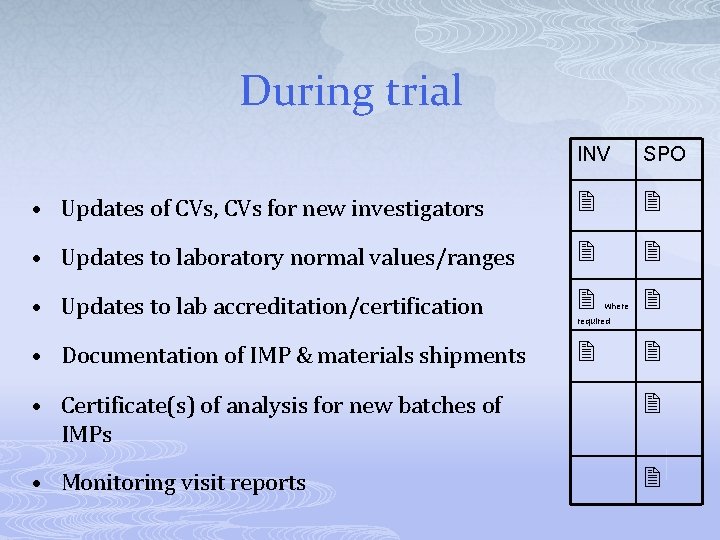
During trial INV SPO • Updates of CVs, CVs for new investigators • Updates to laboratory normal values/ranges • Updates to lab accreditation/certification • Documentation of IMP & materials shipments where required • Certificate(s) of analysis for new batches of IMPs • Monitoring visit reports

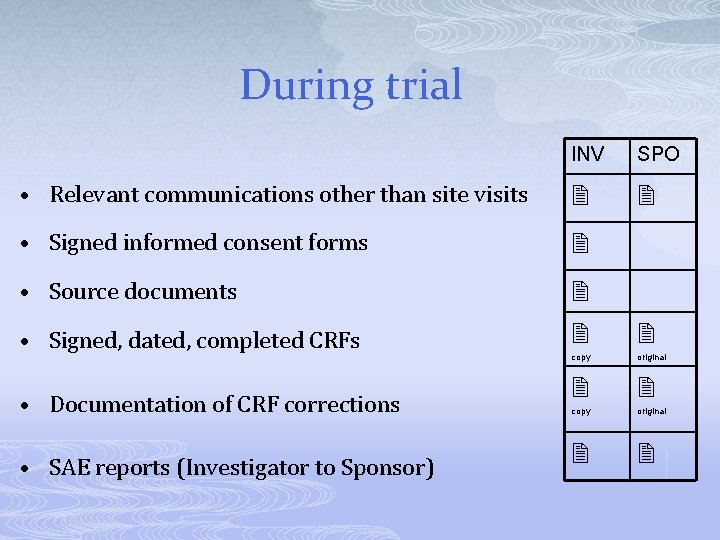
During trial INV SPO • Relevant communications other than site visits • Signed informed consent forms • Source documents copy original • Signed, dated, completed CRFs • Documentation of CRF corrections • SAE reports (Investigator to Sponsor)

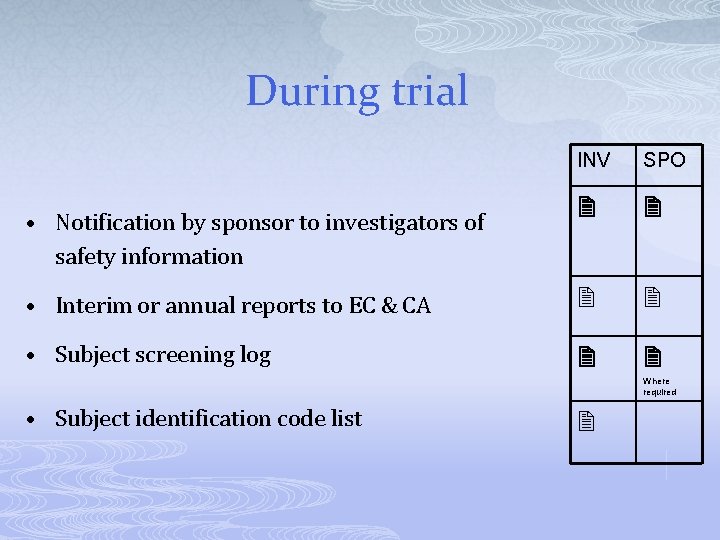
During trial INV SPO • Interim or annual reports to EC & CA • Subject screening log • Notification by sponsor to investigators of safety information Where required • Subject identification code list

During trial INV SPO • IMP accountability at site • Signature sheet • Record of retained body fluids/tissue samples (if any)

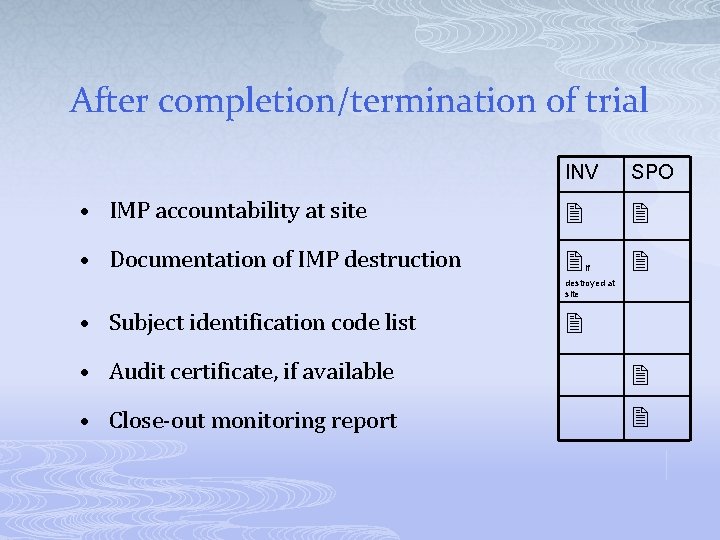
After completion/termination of trial INV SPO • IMP accountability at site • Documentation of IMP destruction if destroyed at site • Subject identification code list • Audit certificate, if available • Close-out monitoring report

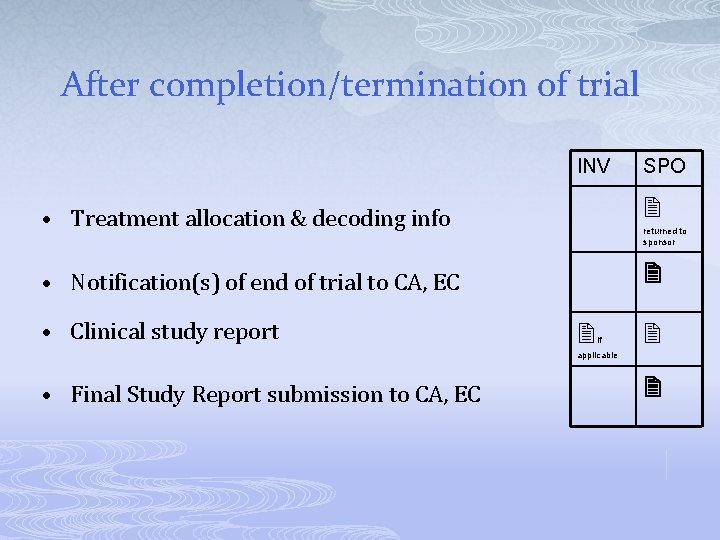
After completion/termination of trial INV SPO • Treatment allocation & decoding info • Notification(s) of end of trial to CA, EC • Clinical study report returned to sponsor if applicable • Final Study Report submission to CA, EC

Objectives p p p Developing/Writing a protocol. Developing an Investigator Site File (ISF) – Regulatory Binder. Screening, Recruitment, Enrollment and Retention.

Patient Recruitment Determining the best way to recruit for a particular study requires experience plus an understanding of the recruitment process.

Planning p Determine who will be involved? p Discuss multiple strategies p Establish goals and timelines p Develop recruitment materials n p Ads, brochures, educational materials Plan to be flexible

Enrolment p p Enroll only individuals who meet ALL of the Eligibility Criteria. Using individuals that do not meet each of the inclusion and exclusion criteria constitutes a protocol violation.

Barriers to Recruitment and Retention p Subject-related barriers p Investigator-related barriers p Protocol-related barriers p Other barriers

Subject Barriers p p p Long clinic waiting times Inconvenient appointment scheduling Dislike of uncertainty associated with the trial; prefer the doctor to make the decision about their treatment Perceived risks outweigh benefits Unrealistic expectations of the clinical trial Site accessibility barriers

Investigator Barriers p p Lack of enthusiasm for the design or aims of the study protocol Lack of time to recruit due to the investigator’s clinical workload and other duties Conflict of roles between caregiver and clinical investigator Investigator involved in too many clinical trials

Protocol Barriers p p p Eligibility criteria that are so tight that potential study subjects do not qualify for entry Protocol too difficult to follow due to complex study designs Lengthy study periods or excessive visit schedules

Other Barriers p Negative influence of the media p Social stigma associated with the research p Lengthy ethical approval process may delay recruitment and trial commencement p Multiple studies competing for same patients p Lack of referrals from colleagues to the clinical trial p Poor choice of study site by the sponsor n n Inaccurate estimate of patient population Not enough staff resources for the site

Methods for Patient Retention p p p Don’t recruit “doubtful” patients Determine availability to attend visits Get as many contact details as possible: friends, family, caregiver, employer, usual medical practitioner

Methods for Patient Retention (cont. ) Transportation money p Be flexible p p Dignity and respect

Methods for Patient Retention (cont. ) § § § Clean and comfortable waiting area Tea, coffee, sandwiches Make patient feel special

Methods for Patient Retention (cont. ) p p Serious adverse events – explain and make sure patient understand what is going on Always encourage communication by phone, email, letters Home-visits End of year party

THANK YOU Tamer Hifnawy MD. Dr PH. Associate Professor of Public Health & Community Medicine Faculty of Medicine, Beni Suef University, Egypt College of Dentistry Taibah University, KSA Certified Trainer on Ethics of Human Research Consultant Email: tamer. hifnawy@med. bsu. edu. eg thifnawy@taibahu. edu. sa thifnawy@yahoo. com Mobile: +201114130107 Egypt +966564356123 KSA