Clinical Trial Unit Avdeling forskningssttte for kliniske studier












- Slides: 21

Clinical Trial Unit Avdeling forskningsstøtte for kliniske studier OUS/OSS/FST/CTU NORM Meeting Oslo 17 Sep 2019 Jon B Borgaard, Head of department, CTU

Research Support at OUH • Facilitate research • Locally • Regionally • Nationally • Provide expert knowledge • Ensure infrastructure for large clinical trials of good quality • Provide support for (almost) all stages of the research process from idea to publication – and to implementation of new treatment

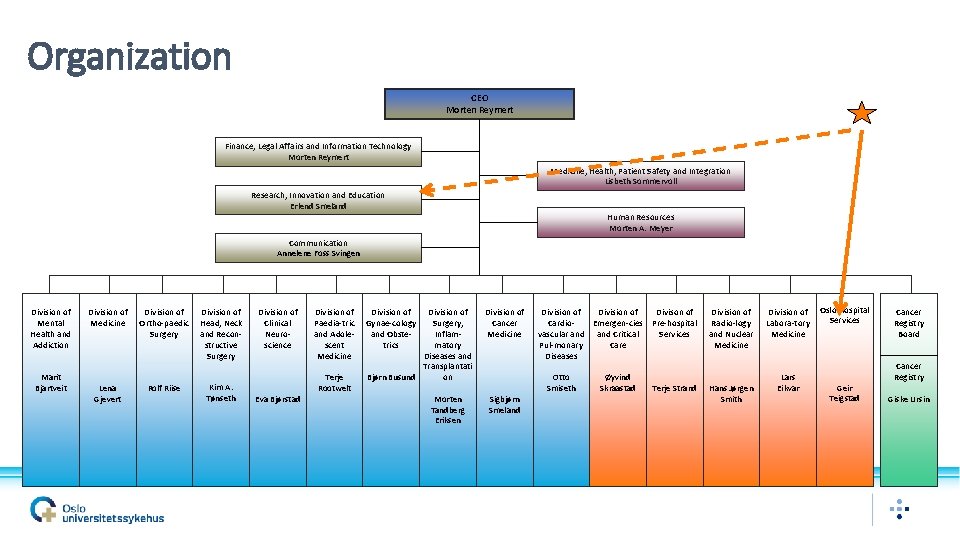
Organization CEO Morten Reymert Finance, Legal Affairs and Information Technology Morten Reymert Medicine, Health, Patient Safety and Integration Lisbeth Sommervoll Research, Innovation and Education Erlend Smeland Human Resources Morten A. Meyer Communication Annelene Foss Svingen Division of Mental Health and Addiction Marit Bjartveit Division of Medicine Lena Gjevert Division of Ortho-paedic Surgery Rolf Riise Division of Head, Neck and Reconstructive Surgery Kim A. Tønseth Division of Clinical Neuroscience Eva Bjørstad Division of Paedia-tric and Adolescent Medicine Terje Rootwelt Division of Surgery, Inflammatory Diseases and Transplantati on Bjørn Busund Division of Gynae-cology and Obstetrics Morten Tandberg Eriksen Division of Cancer Medicine Sigbjørn Smeland Division of Divison of Cardio. Emergen-cies Pre-hospital vascular and Critical Services Pul-monary Care Diseases Otto Smiseth Øyvind Skraastad Terje Strand Division of Radio-logy and Nuclear Medicine Hans Jørgen Smith Division of Labora-tory Medicine Lars Eikvar Oslo Hospital Services Cancer Registry Board Cancer Registry Geir Teigstad Giske Ursin

Vision A CTU should contribute to an increased number of high quality clinical studies initiated by researchers at OUS, and should strengthen our position as an internationally outstanding university hospital. A CTU should facilitate clinical studies at other hospitals in the South-Eastern Norway Regional Health Authority and contribute to a high quality healthcare system meeting future requirements in the Region. (Memo by Kristin Bjordal to the OSS Director, Research Director in OUS and SENRHA, 20 Dec. 2016)

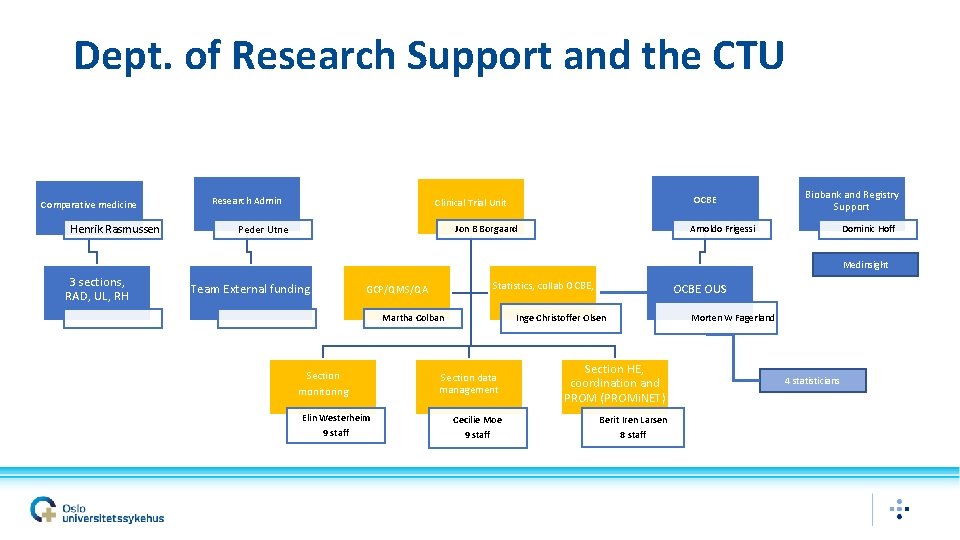
Dept. of Research Support and the CTU Comparative medicine Henrik Rasmussen Research Admin OCBE Clinical Trial Unit Peder Utne Jon B Borgaard Biobank and Registry Support Arnoldo Frigessi Dominic Hoff Medinsight 3 sections, RAD, UL, RH Team External funding Statistics, collab OCBE, GCP/QMS/QA Martha Colban OCBE OUS Inge Christoffer Olsen Morten W Fagerland Section data management Section HE, coordination and PROM (PROMi. NET) Elin Westerheim Cecilie Moe Berit Iren Larsen 9 staff 8 staff Section monitoring 4 statisticians

How can the CTU help? • Contracted services • Monitoring of clinical trials according to ICH-GCP • Data Management, including CRF design, database design and data validation • Statistics, trial design, sample size evalulation, randomization, analysis • Courses • GCP, HE, PROM • Advicing • GCP, monitoring, statistics, data management, health economy, PROM, etc. • Development and quality assurance of internal hospital procedures, particularly with respect to pharmaceutical clinical trials

Project coordination • Coordinate requests to the CTU • Help identify the need for other support functions in Research support services • Participate in initial meeting with new projects • Project coordination for dedicated studies contracted to CTU.

Monitoring • Mandatory for pharmaceutical trials • Work according to the Nor. CRIN SOPs • Experienced team • Development topics • Risk based monitoring • Central monitoring

Data Management • e. CRF solution for clinical trials (Viedoc) • • • Design according to protocol Data validation Coding Randomisation Delivery of data sets • Advising on data management in general

Health Economics • 4 specialists • Advising and guidance for researchers and research fellows • Courses and training • Contrated services in new CTU trials (soon. . )

PROMi. NET • PROMi. NET: A Regional Infrastructure for Patient Reported Outcome Measures in Clinical Research • Secretariat organised under CTU • Chairman, vice-chairman, project coordinator • Courses and guidance • Advising in new CTU trials if relevant

Special adviser GCP • Advising and training on GCP and guidelines • Maintains hospital procedures • Procedure governing all research at OUS (Forskningsprosedyren) • Roles and responsibility in pharmaceutical clinical trials and medical device studies • • Nor. CRIN WP#2 (SOPs) and WP#3 (Monitoring) Participate in initial meetings for new trials Protocol GCP review GCP training courses at OUH and other hospitals

Special adviser Statistics • Advising and training on statistics and study design • Participate in initial meetings for new trials • Primary contact and “line management responsibility” for OCBE Statisticians assigned to CTU studies • Study Statistician on assigned projects – planning, conduct, reporting • Nor. CRIN – establish new work package for statistics

CTU Funding • The basic funding from OUH and SENRHA is fixed. • CTU growth should be funded from increased payment from projects/studies. (Similar to all other support functions; Radiology, Labs, . . ). • CTU is not allowed to subsidise any project Contracted services OUH base og SENRHA long term project Time

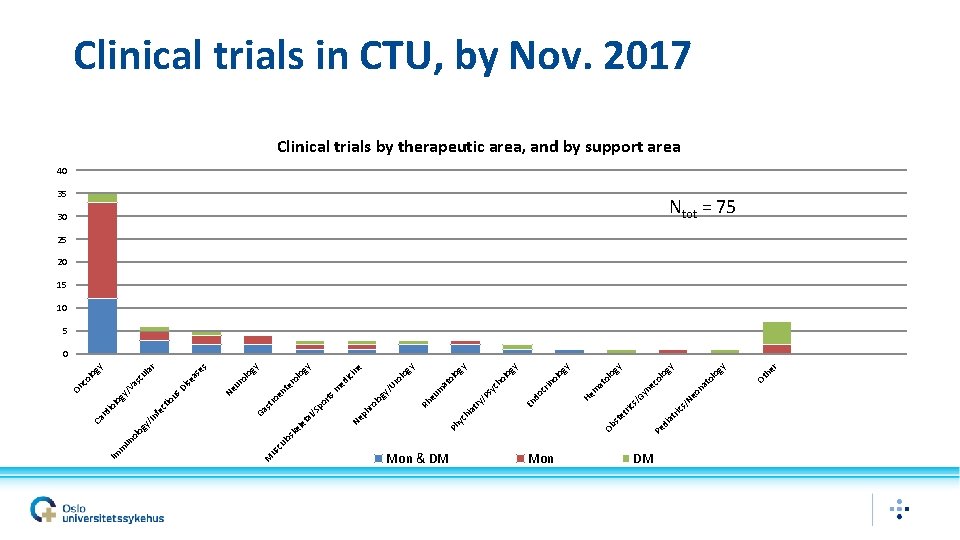
CTU Bits & Pices • Not mandatory to use CTU • CTU Quality stamp: Ensure GCP is followed • Jun 2019 - 146 trials ongoing • Sponsor OUS = 72, Regional = 22, Other National = 11, International = 41 • 2019 budget • • • Total budget OUS base Regional funding Contracted services Other project 27. 8 MNOK 1. 7 MNOK 17. 0 MNOK 4. 5 MNOK 4. 6 MNOK

Mon & DM Mon DM r he Ot y 30 og 35 ol at on Ne gy lo co y og ol at ne Gy ric s/ at di Pe et st Ob m He ol og y rin ol og y y og ol at ch sy oc /P En d ry at m eu Rh y ne og ol Ur ol og y/ ph r gy gy lo ro ed ici m te en rts po /S yc hi Ph Ne al et el lo sk st ro Ga lo ur o se s se a Di Ne us r la gy lo sc u Va gy / lo ct io fe In un ol og y/ cu M us m Im io rd Ca On co Clinical trials in CTU, by Nov. 2017 Clinical trials by therapeutic area, and by support area 40 Ntot = 75 25 20 15 10 5 0

Cooperation • International working group for monitors, NORM • Member of the Nor. CRIN 1 and Nor. CIRN 2 project (www. norcrin. no) and several of its working packages (monitoring, project coordination, etc. )

Nor. CRIN • Project, 6 univ hospitals are partners • SOPs, templates for clinical trials and other intervensions • …and a lot of other valuable info

Contact information • E-mail (mailbox): • Research support: OUSHF PB CTU oushfpbctu@ous-hf. no • Internet: • https: //oslo-universitetssykehus. no/fag-og-forskning/regionalforskningsstotte/forskningsstøtte-for-kliniske-studier----clinical-trial-unit-(ctu) (or search on «www. oslo-universitetssykehus. no and CTU» )


Many thanks for your attention jobbor@ous-hf. no