Clinical Trial Proposal Presentation Template for open forum











- Slides: 12

Clinical Trial Proposal Presentation Template for open forum at the 2017 ASM

Title Investigators and sites

Rationale • Is there a high quality systematic review that indicates this is an evidence gap? • Are there any published or ongoing similar trials (and if so, explain why another trial is needed)?

Aims and study design What is the research question? Specify the PICO (population, intervention, control, outcomes) Specify the study design

Participants Inclusion criteria Exclusion criteria

Interventions Active group Control group

Outcome measurement Include who will perform assessments Baseline measures Timing of follow up Outcome measures (include scale, MICD)

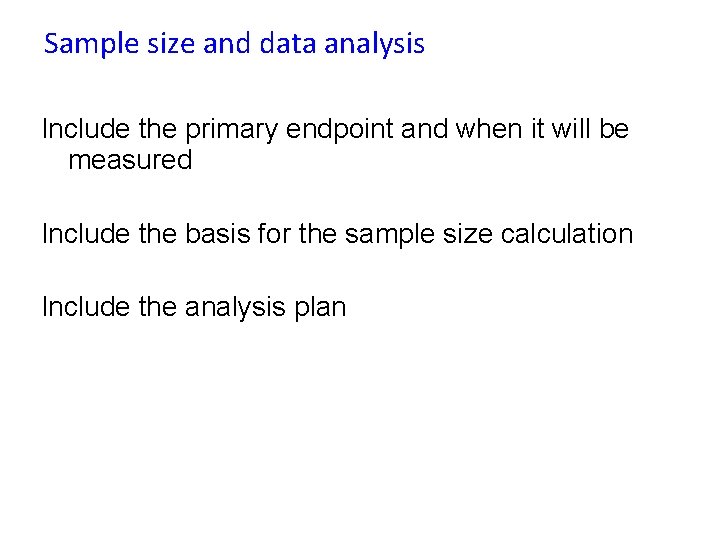
Sample size and data analysis Include the primary endpoint and when it will be measured Include the basis for the sample size calculation Include the analysis plan

Feasibility • Comment on the trial feasibility and whether or not you think pilot data are needed • Is the trial feasible (reflecting cost, logistics, track record and likely recruitment rate)?

Implementation If the trial indicates a clinical practice or other change is desirable, are there any possible barriers to implementation? Does the trial have the potential to change practice and/or policy?

CONSIDER THESE ANZMUSC ENDORSEMENT CRITERIA • Does the research question concern an important disease burden and/or an important evidence or evidence-practice gap? • What is the evidence that the research question is one that the clinical and/or consumer community need answered? • Does answering the research question have strong potential to change practice and/or policy (reflecting academic impact, implementation, and generalisability)?

CONSIDER THESE ANZMUSC ENDORSEMENT CRITERIA • Are the methods of high quality and does the proposal include an economic evaluation and process measures where relevant? • Is the trial multicentre (to encourage collaboration, increase power and increase implementation of findings)?