Clinical trial Data Programming Management What can Exe












- Slides: 24

Clinical trial Data Programming & Management What can Exe. CTU do for you?

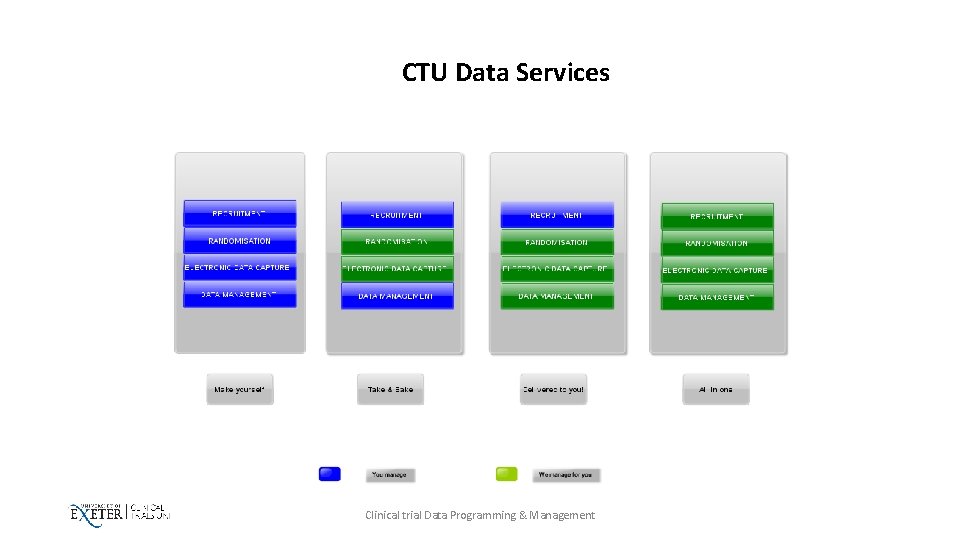
What we can help you with? • Recruitment • Randomisation • Electronic Data Capture • Data management Clinical trial Data Programming & Management

SPA Case Study Clinical trial Data Programming & Management

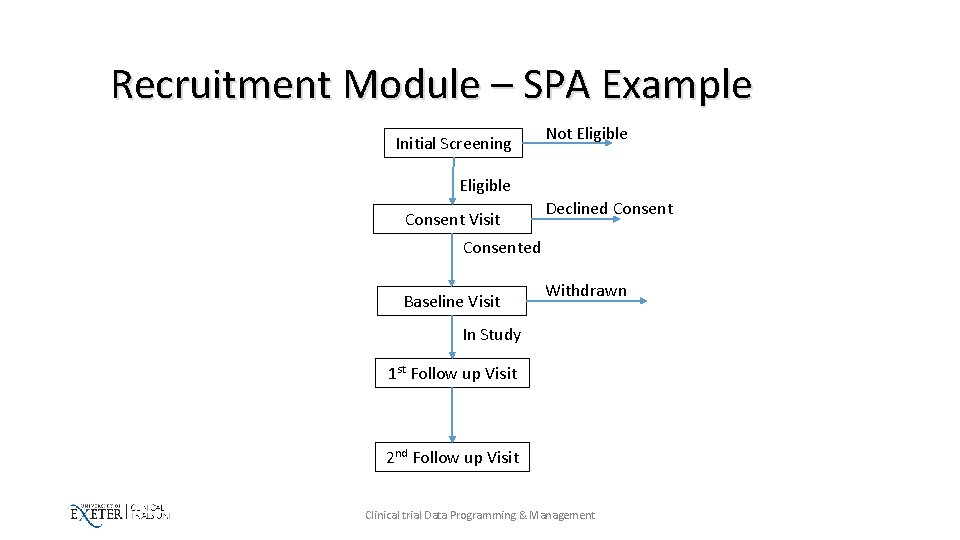
Recruitment Module – SPA Example Initial Screening Not Eligible Consent Visit Declined Consented Baseline Visit Withdrawn In Study 1 st Follow up Visit 2 nd Follow up Visit Clinical trial Data Programming & Management

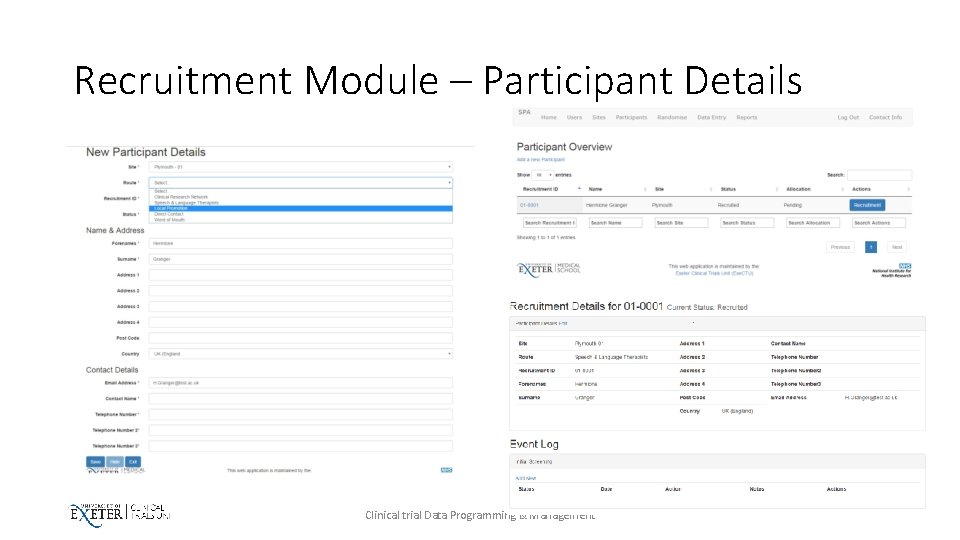
Recruitment Module – Participant Details Clinical trial Data Programming & Management

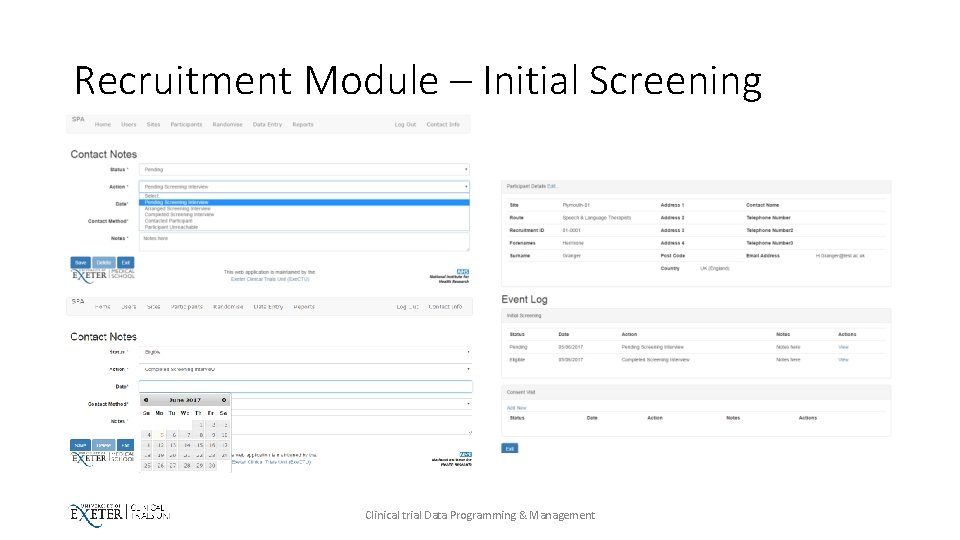
Recruitment Module – Initial Screening Clinical trial Data Programming & Management

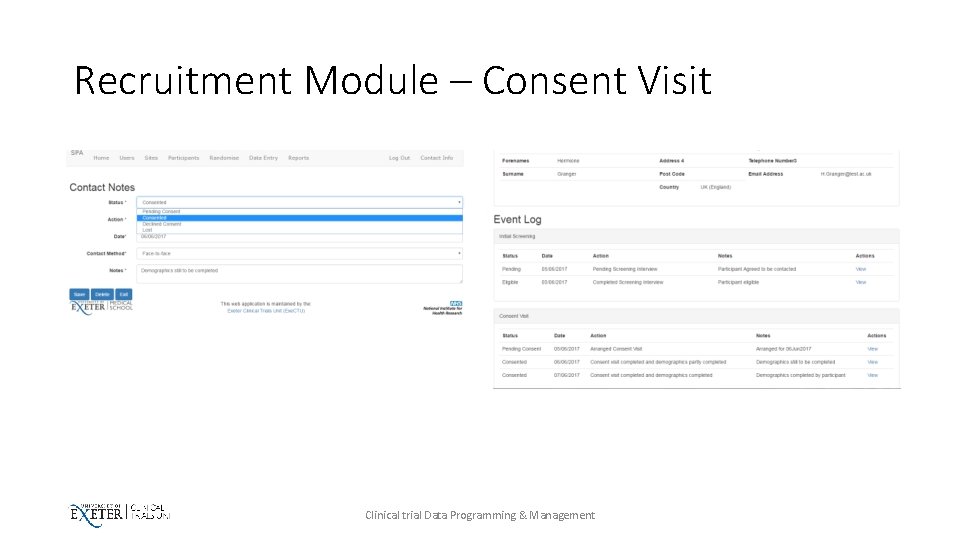
Recruitment Module – Consent Visit Clinical trial Data Programming & Management

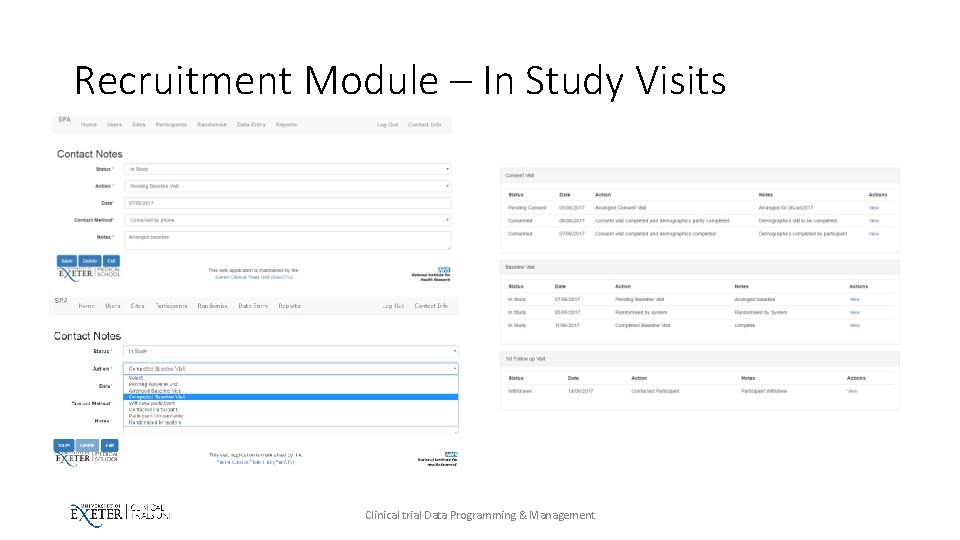
Recruitment Module – In Study Visits Clinical trial Data Programming & Management

Randomisation • Pure random • Static block • Dynamic block • Minimisation Clinical trial Data Programming & Management

Electronic Data Capture • Bespoke Case Report Form • Tele-Form - paper scanning system • Systems validation (levels) • Enterprise Electronic Data Capture System (out to tender) Clinical trial Data Programming & Management

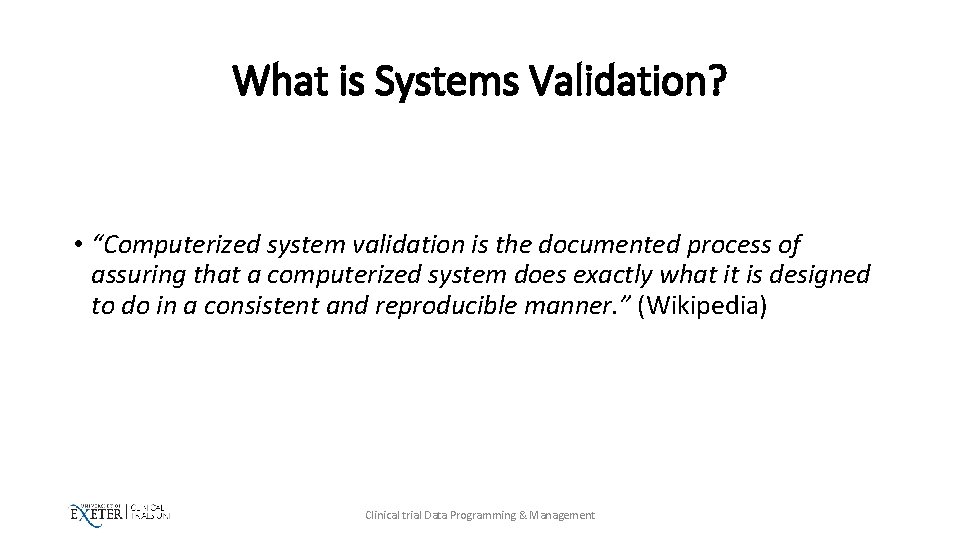
What is Systems Validation? • “Computerized system validation is the documented process of assuring that a computerized system does exactly what it is designed to do in a consistent and reproducible manner. ” (Wikipedia) Clinical trial Data Programming & Management

The system does exactly what it is designed to do in a consistent and reproducible manner Clinical trial Data Programming & Management

Why bother? • It is a requirement for CTIMPS - "MHRA deficiency reporting trends show that failure to perform Computerised System Validation is classed as a Major Observation". • It is a recommendation for GCP - "Systems with procedures that assure the quality of every aspect of the trial should be implemented". • It is best practice -"Doing things properly saves time, reduces errors and the chance of harming research, or people". Clinical trial Data Programming & Management

What does it mean for you? • Formal process • • • User requirements User acceptance testing Sign off Change management Faster Development • Peace of mind, avoiding breeches and unnecessary amendments • Does exactly what is says on the tin! • . . . And proof for your paper! Clinical trial Data Programming & Management

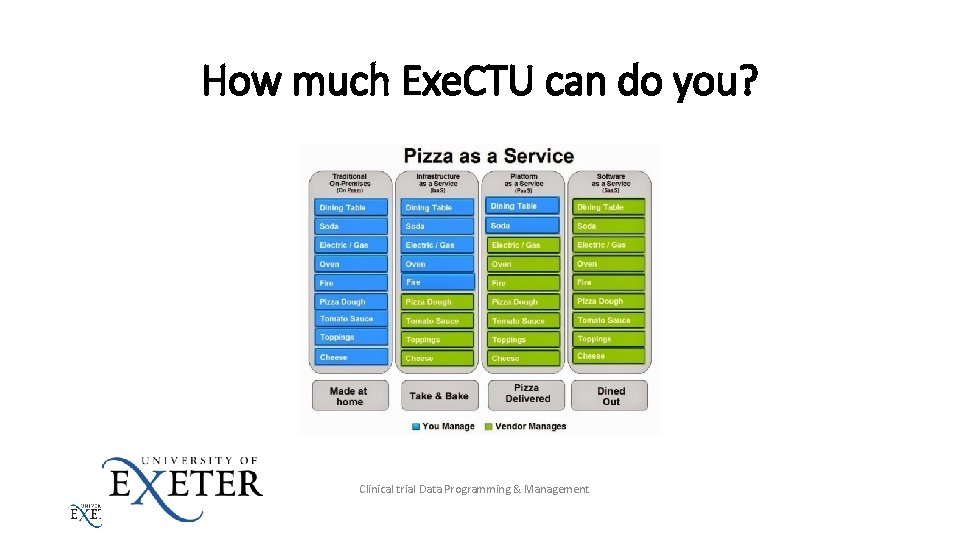
How much Exe. CTU can do you? Clinical trial Data Programming & Management

CTU Data Services Clinical trial Data Programming & Management

Data Management • Working with data outside of programming tasks • Some R/Stats data tasks for export & analysis • Data Security & Risk Analysis • Data Management Plan • Meta Analysis • • Data storage Co-ordination in obtaining datasets Data Cleaning Collaboration with the Statistical team Clinical trial Data Programming & Management

INTERPRESS-IPD Case Study Clinical trial Data Programming & Management

INTERPRESS-IPD The Inter-arm blood pressure difference individual patient data collaborations

INTERPRESS-IPD The project seeks to develop a new prognostic model for cardiovascular risk estimation that includes IAD

INTERPRESS-IPD • Is an NIHR Research for Patient Benefit Programme • Is an International collaboration Study • Combines individual patient data (IPD) from IAD studies • Cleaning Data • One large dataset • Meta-analysis • perform cross-sectional analyses to describe the epidemiology of IAD in the dataset. • Lead investigators contributing datasets will be acknowledged in all relevant publications. Clinical trial Data Programming & Management

INTERPRESS-IPD Clinical trial Data Programming & Management

Where we’re heading. . . • Enterprise EDC tool frees up programmer time for: • Comprehensive Document Management System – Share. Point • SOPS, Policies & Work Instructions • Review & Approval Workflows • Electronic Trial Master Files • Electronic Archiving • Advanced Recruitment & Randomisation modules • Systems Integration & Big Data • Statistical Programming • Advanced STATA / SPSS • R Clinical trial Data Programming & Management

CTU data team ctudata@exeter. ac. uk 01392 40 6793 Clinical trial Data Programming & Management