CLINICAL TRIAL Clinical Trials Strengths Best measure of

CLINICAL TRIAL

Clinical Trials Strengths: – Best measure of causal relationship – Best design for controlling bias – Can measure multiple outcomes Weaknesses: – High cost – Ethical issues may be a problem – Compliance

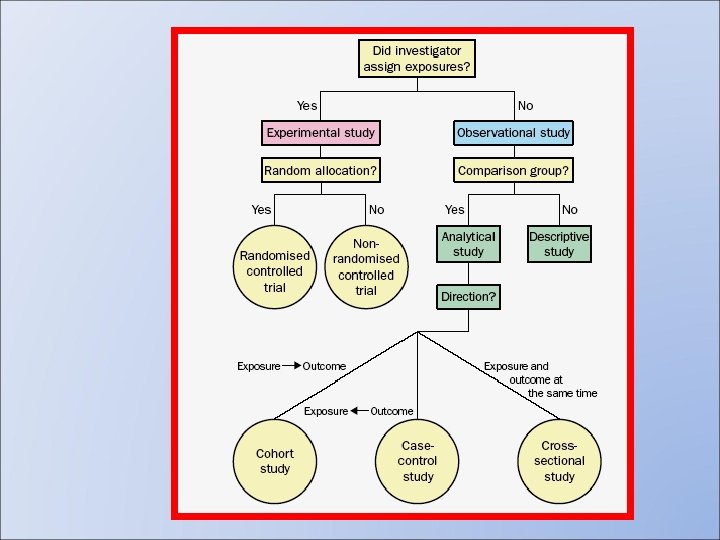
Intuition and Logic in Research Intuition Dominant Mental Activity Analysis Feeling Judgeme nt Experien ce Qualitative Research Hi Clinical Cohorttrials Study Case-control Study Crosssectional Study Case Report Experiment Hi Control over varianc e Case Series Lo Potential for Misinterpretation Lo

Randomised Controlled Trial (RCT)

Strength of evidence Experimental Systematic Review RCT Observational Prospective Retrospective Anecdote Cohort study Case-control study Case series

Randomised Controlled Trial (RCT) RCT is a trial in which subjects are randomly assigned to two groups: -the experimental group -the comparison group or Controls Source: Cochrane Collaboration Glossary
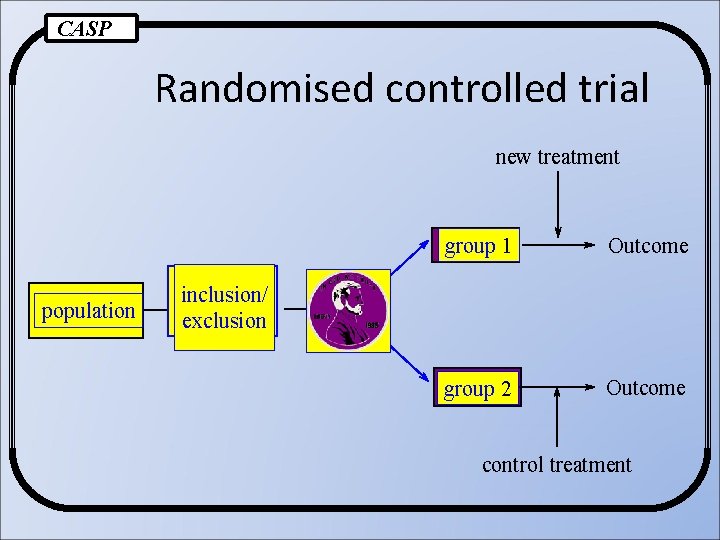
CASP Randomised controlled trial new treatment population group 1 Outcome group 2 Outcome inclusion/ exclusion control treatment

Ø Study population (participant) treatment / control Ø Investigators Ø Assessors Ø Clinical intervention (medical, surgical, hygiene) Ø Outcome

Who is in control? • Every experiment should have a “control group. ” • People in control group are treated exactly the same way as the other people in the experiment, except they do not get the “active treatment. ” • A “placebo group” is a special kind of control group.

RANDOMIZATION Definition advantage Pseudo randomization( quasi –R) disadvantage


Blinding: Open Single-blind Double blind : with placebo or active control(double dummy) neither the researcher nor the individuals know who received what Triple blind
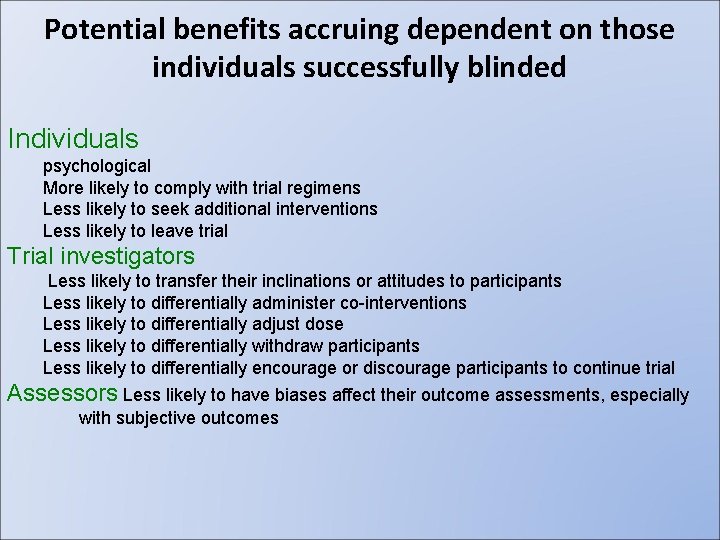
Potential benefits accruing dependent on those individuals successfully blinded Individuals psychological More likely to comply with trial regimens Less likely to seek additional interventions Less likely to leave trial Trial investigators Less likely to transfer their inclinations or attitudes to participants Less likely to differentially administer co-interventions Less likely to differentially adjust dose Less likely to differentially withdraw participants Less likely to differentially encourage or discourage participants to continue trial Assessors Less likely to have biases affect their outcome assessments, especially with subjective outcomes

Ascertainment selection BIAS publication Inappropriate handling of withdrawals

• SELECTION BIAS Inclusion & exclusion Intervention New drug on MS and depression • Randomization • Allocation concealment – if both patients and investigators could not predict the next assignment of treatment


Ø Double blinding prevents ascertainment bias and protects randomization after allocation and during study Ø Allocation concealment prevents selection bias and protects randomization during selection

RCT IS NOT suitable for: * ETIOLOGY AND CLINICAL COURSE smoking and cancer * RARE & PROLONGED OUTCOME

ethics • Phase 1 – 20 -80 – Toxic and pharmacologic effects • Phase 2 – 100 -200 – Efficacy – immunity • Phase 3 – RCT – Multicenter • Phase 4 – After release

Quality of RCT

RCTs - a checklist • • • Good randomisation procedures patients blind to treatment clinicians blind to treatment all participants followed up all participants analysed in the groups to which they were randomised (intention to treat)

limitations • Loss to follow up • Contamination – Drop out – Drop in

Effect

Randomized Clinical Trials Cure Yes No Total A 13 87 100 B 25 75 100 38 162 200 Treatment

ARR(absolute risk reduction) RR OR RRR: Efficacy= (risk in treatment-risk in control)/risk in control • NNT(Number needed to treat)=1/ARR • •

Definition • Number Needed to Treat (NNT): – Number of persons who would have to receive an intervention for 1 to benefit. – 100/ARR (where ARR is %) – 1/ARR (where ARR is proportion)
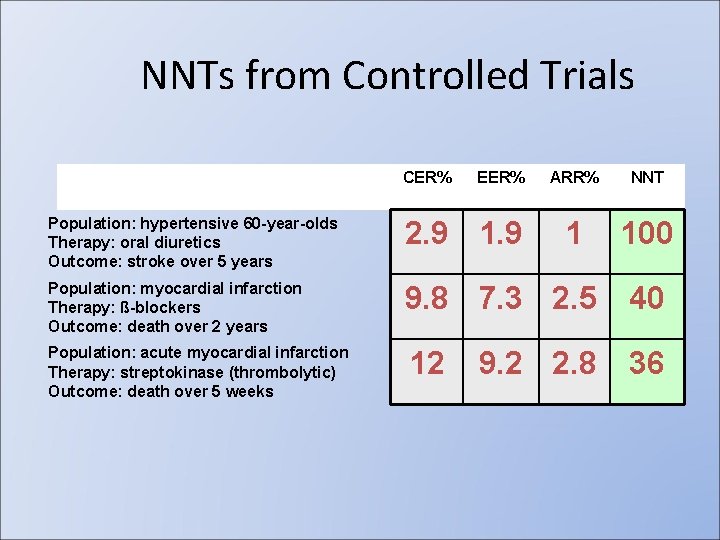
NNTs from Controlled Trials CER% EER% ARR% NNT 1 100 Population: hypertensive 60 -year-olds Therapy: oral diuretics Outcome: stroke over 5 years 2. 9 1. 9 Population: myocardial infarction Therapy: ß-blockers Outcome: death over 2 years 9. 8 7. 3 2. 5 40 Population: acute myocardial infarction Therapy: streptokinase (thrombolytic) Outcome: death over 5 weeks 12 36 9. 2 2. 8
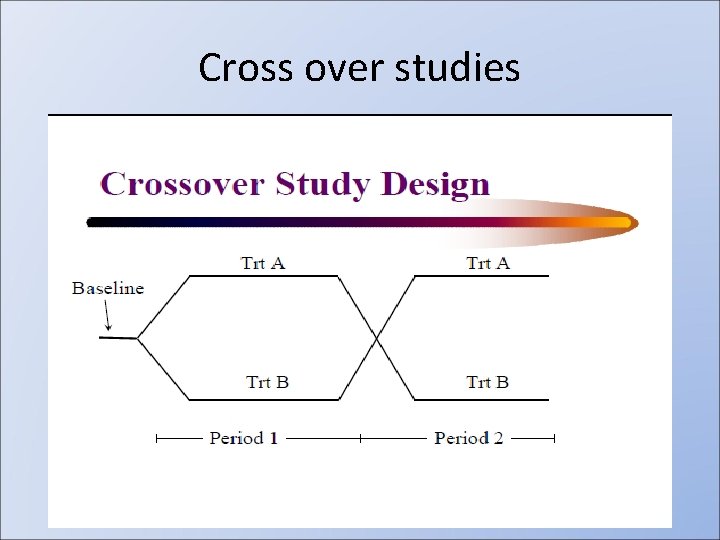
Cross over studies

Cross over studies • Types: – planned • Washout period • Sequence of treatment – Unplanned
Factorial designs • Two or more independent variables are manipulated in a single experiment • They are referred to as factors • The major purpose of the research is to explore their effects jointly • Factorial design produce efficient experiments, each observation supplies information about all of the factors 37
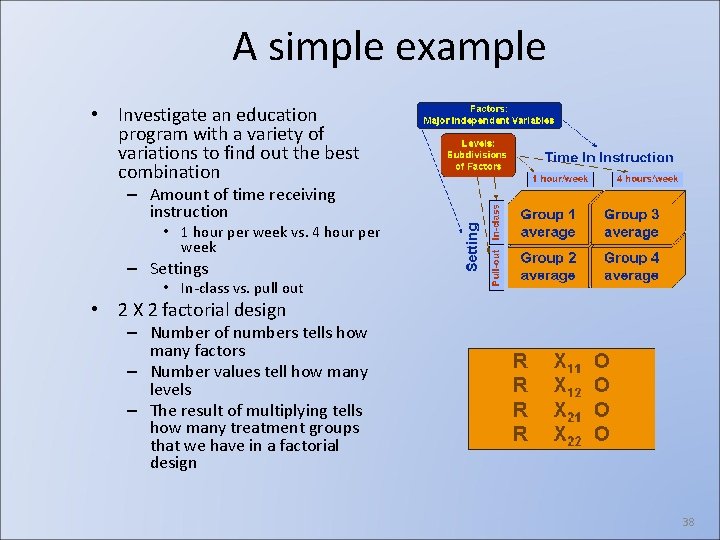
A simple example • Investigate an education program with a variety of variations to find out the best combination – Amount of time receiving instruction • 1 hour per week vs. 4 hour per week – Settings • In-class vs. pull out • 2 X 2 factorial design – Number of numbers tells how many factors – Number values tell how many levels – The result of multiplying tells how many treatment groups that we have in a factorial design 38

Main effects • Main effect of time • Main effect of setting • Main effects on both 40
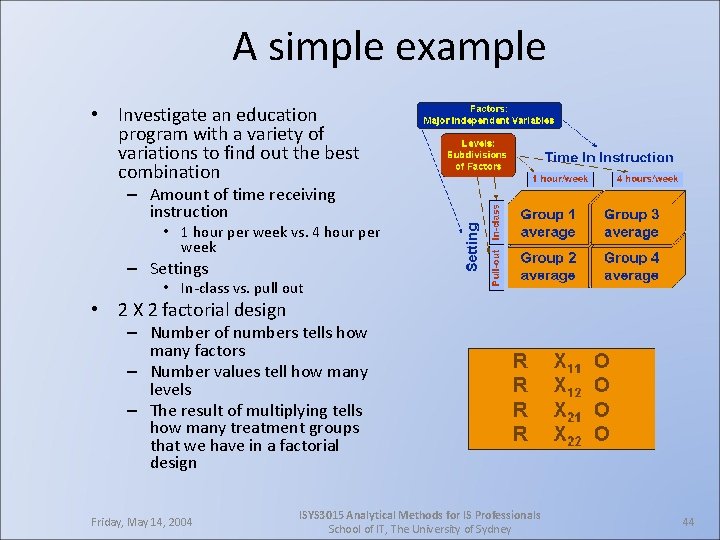
A simple example • Investigate an education program with a variety of variations to find out the best combination – Amount of time receiving instruction • 1 hour per week vs. 4 hour per week – Settings • In-class vs. pull out • 2 X 2 factorial design – Number of numbers tells how many factors – Number values tell how many levels – The result of multiplying tells how many treatment groups that we have in a factorial design Friday, May 14, 2004 ISYS 3015 Analytical Methods for IS Professionals School of IT, The University of Sydney 44
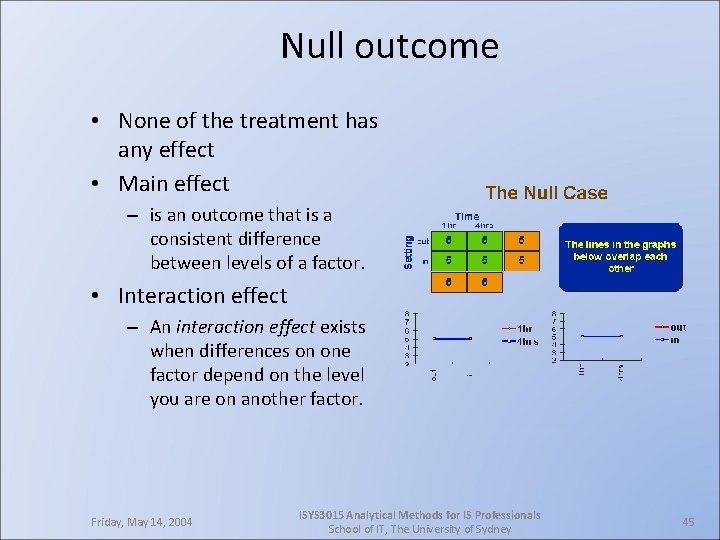
Null outcome • None of the treatment has any effect • Main effect – is an outcome that is a consistent difference between levels of a factor. • Interaction effect – An interaction effect exists when differences on one factor depend on the level you are on another factor. Friday, May 14, 2004 ISYS 3015 Analytical Methods for IS Professionals School of IT, The University of Sydney 45

Main effects • Main effect of time • Main effect of setting • Main effects on both Friday, May 14, 2004 ISYS 3015 Analytical Methods for IS Professionals School of IT, The University of Sydney 46

Interaction effect • An interaction effect exists when differences on one factor depend on the level of another factor Friday, May 14, 2004 ISYS 3015 Analytical Methods for IS Professionals School of IT, The University of Sydney 47

Interaction effect • Interaction as a difference in magnitude of response • Interaction as a difference in direction of response Friday, May 14, 2004 ISYS 3015 Analytical Methods for IS Professionals School of IT, The University of Sydney 48
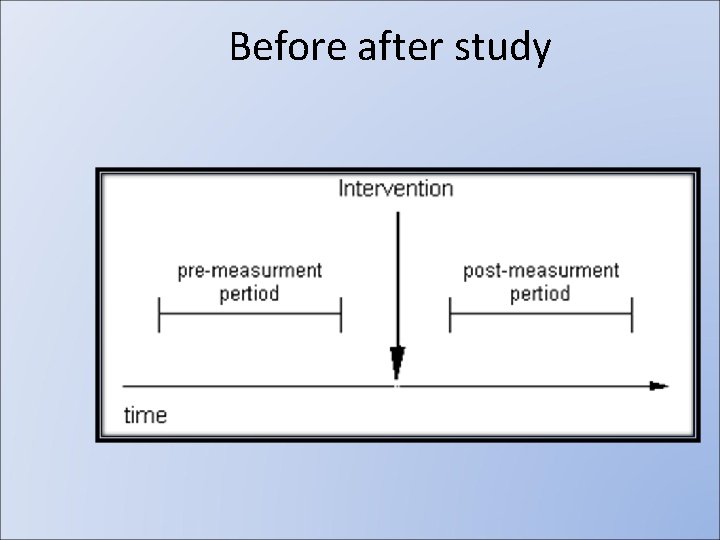
Before after study
- Slides: 39




























































