Clinical Research in the Emergency Department Jim Quinn














































- Slides: 54

Clinical Research in the Emergency Department Jim Quinn MD MS Associate Professor of Surgery/Emergency Medicine Research Director Emergency Medicine

Overview • Goals for research in academic emergency medicine • Problems/solutions for researchers in emergency medicine • Problems/solutions for research implementation • The new clinical research unit in the ED at Stanford

Academic Emergency Medicine • Outstanding residency and clinical operations • Research lags behind educational and clinical performance • Excellence in education, clinical operation and research will lead to departmental status at the university • Departmental status will lead to more academic and fiscal freedom

Improving Research How Do We Get There • - Obstacles Too busy No training in research Too few mentors/role models No interest

Improving Research How Do We Get There • Solutions 1) Recruit researchers 2) Develop researchers - Personal and academic investment - Expose EM residents to advantages of academic career - Funding through grants and career development awards

Research During Residency • Train residents to appreciate research efforts, critically evaluate a study - Study design, methodology, statistics • Exposure to opportunities for an academic career • Some will decide to do research fellowships and pursue academic medicine

Research During Residency • • • - Start early (1 st year) Develop own idea Develop that idea with a faculty mentor avoid doing research for them find mentors with common interest Research curriculum and support to facilitate project development • Research Director- support and direction

Research Curriculum Structure and Support to Develop Your Idea • Curriculum (tried and tested) - 12 hours – 6 sessions with core reading and homework designed to develop your project • Textbook - “Designing Clinical Research” - Hulley and Cummings - small paperback readable • Help identify mentors and sources of data

The Five Page Protocol Goal for the Research Curriculum • Concise protocol - More concise than an NIH submission, but often sufficient for small intramural grants - Discipline approach to planning the study - Provide the materials and answers for IRB submission - Completed by the end of first year - Implement in years 2 and 3

Organization The Five Page Protocol • Page One - Title, Specific objectives, significance • Pages 2 -5 - Overview of design (RCT, observational cohort/ cross sectional, case/control) - Study subjects: selection criteria, exclusions, accessible populations, plans for sampling and recruitment - Measurement – predictor and outcome variables - Statistical issues – sample size, proposed analysis - Quality control and data management - Timetable - Ethical considerations

Research Development • Residents - EMF: Resident Research Grants - $5, 000 - EMF, SAEM: Research Fellowships - $75, 000 - T and F awards from NIH Faculty - Career development awards SAEM, EMF, K awards from NIH

What is a Career Development Award ? Funding to protect your time so that you can develop your research skills Research may be: Clinical Basic Science

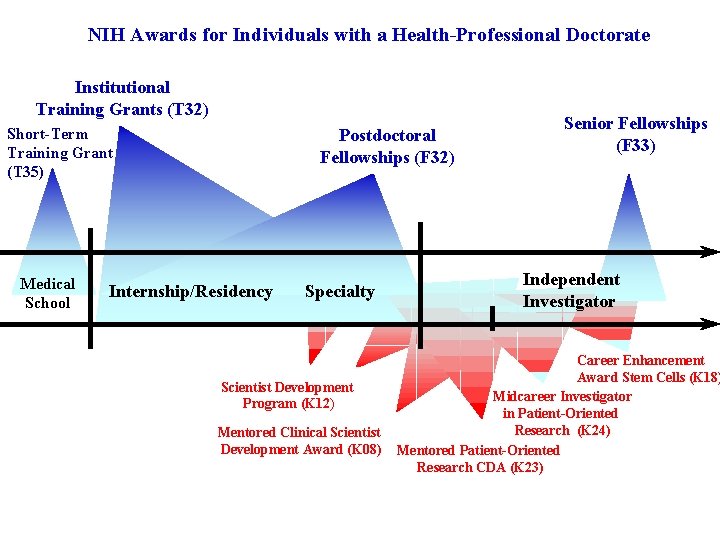
NIH Awards for Individuals with a Health-Professional Doctorate Institutional Training Grants (T 32) Short-Term Training Grant (T 35) Medical School Postdoctoral Fellowships (F 32) Internship/Residency Specialty Scientist Development Program (K 12) Mentored Clinical Scientist Development Award (K 08) Senior Fellowships (F 33) Independent Investigator Career Enhancement Award Stem Cells (K 18) Midcareer Investigator in Patient-Oriented Research (K 24) Mentored Patient-Oriented Research CDA (K 23)

Benefits of a Career Development Award ? • • Protected time Extra Training Step towards independence New relationships

Myth: NIH Grants/Study Sections • Emergency medicine proposals, especially clinical research, will not be evaluated fairly, nor will they be funded consistently, until the NIH has a study section devoted to emergency medicine. • NIH grants are rare and hard to get

What is Important to Study Section Members? • Study section members don’t care what department the investigator is in. • Study section members care about: – The match between the proposed work and the goals of the program – The quality of the proposal – Investigator’s track record and preliminary data – The institutional research environment

Selected NIH Panel Recommendations • “The NIH must ensure fair and effective reviews of extramural grant applications for support of clinical research: panels that review clinical research – (a) must include experienced clinical investigators and – (b) at least 30 -50% of the applications reviewed by these panels must be for clinical research. ”

Selected NIH Panel Recommendations • “The NIH should improve the quality of training for clinical researchers by requiring grantee organizations to provide formal training experiences in clinical research and careful mentoring by experienced clinical investigators. ”



Does NIH Fund EM Research? • A search of currently funded federal grants using the CRISP database and key words “Emergency” yielded 204 new grants in the years 2000 -2002. • Accurate numbers of grants submitted by specialty are difficult to find and interpret. No separate statistics are maintained for Emergency Medicine.

Research Options • • • - Basic Science Translational Research Clinical Research Large database Retrospective reviews Clinical trials/Prospective cohorts

Myth: Large Databases • Large administrative databases contain large amounts of clinically useful information.

Large Databases • In general, large databases are collected: – For non-research purposes (e. g. , claims and billing databases) – With no specific research question in mind (e. g. , trauma center databases)

Large Databases • Large databases often lack the specific outcome and risk stratification variables needed for a particular study, requiring assumptions and approximations to be made. • Large databases often have a substantial proportion of missing or incorrect data which may reflect recording bias or other sources of bias.

Large Databases • Even small biases, together with a large sample size, may yield results with impressively small p values that are, nonetheless, artifacts. • Without independent methods for checking the accuracy and completeness of the data, these biases may be difficult to detect.

Clinical Trials • Prospective trials • Designed to answer specific questions • More likely to answer the question correctly

Problems Implementing Clinical Research • Where did all the patients go? “ The best way to eliminate disease is to study it” • Nobody cares • IRB/HIPAA issues • Department too busy, too many protocols

“Tragedy of the Commons” • A “Metaphor” to describe the sub-optimal use of a collectively shared resource “best strategies for individuals conflict with the common good”

Clinical Research Unit Goal – “conduct efficient and effect research in the chaotic environment of the ED for the common good” - Comprehensive database of all ED patients - Real time data infrastructure - Real time notification and enrollment - Research director, research coordinator, volunteers - Research committee to oversee all projects to ensure adequate resources

Clinical Research Unit Real Time Data Infrastructure - HIPPA complaint ED Research database - Hosted by SOM: secure, redundancy - Allows for instant notification directly from database - Web based enrollment - Eventually paperless - https: //emerg-med. stanford. edu/

Clinical Research Unit Research Coordinator and Volunteers • Volunteers - Undergrads and med students - Help screen and enroll patients - Deal with paper flow • Coordinator - oversees volunteers: schedules - patient follow-up - resource for data and chart acquisition for ED studies.

Clinical Research Unit Research Committee • Meets monthly – 30 -60 minute meetings after faculty meeting 2 nd Wednesday • Open meetings • Oversees and approves all protocols in ED • Consists of research director, resident representation, at least 2 volunteer faculty members

Clinical Research Unit Funding and Resources • Coordinator – 50% time primarily from grant funding • New funding and studies could increase to 100% • Non-EM researchers/industry will have to pay to use our data infrastructure/research unit

Clinical Research Unit Registering Protocols • Send e-mail with protocol to Dr. Quinn • Protocol will be reviewed at research committee for: - IRB approval - Funding Source - Resource Utilization - Benefit to EM - All external protocol will need to have an EM faculty as an investigator/supporter on the protocol

Next Step • Identify current projects utilizing ED patients/resources • Hire coordinator – Completed Dec/Jan • Volunteer recruitment - Ongoing • First committee meeting in December

Clinical Research Unit The First Studies • Dog Bite Study - Requires prospective enrollment of patients and consent • NET-2 - Surveillance study to be part of large NINDS study, no consent

Are Prophylactic Antibiotics Beneficial in Dog Bites? • Controversial 1) Meta analysis- Ann Emerg Med – 1994 - Recommend treating 2) Cochrane Review 2004 - Recommend Not Treating 3) Current recommendation is to treat high risk wounds

Are Prophylactic Antibiotics Beneficial in Dog Bites? • Is it worth doing the study? - Over 1, 000 patients needed in a multi-center trial at great cost to determine a 5% difference (less power on sub group analysis) - Is 5% an important difference?

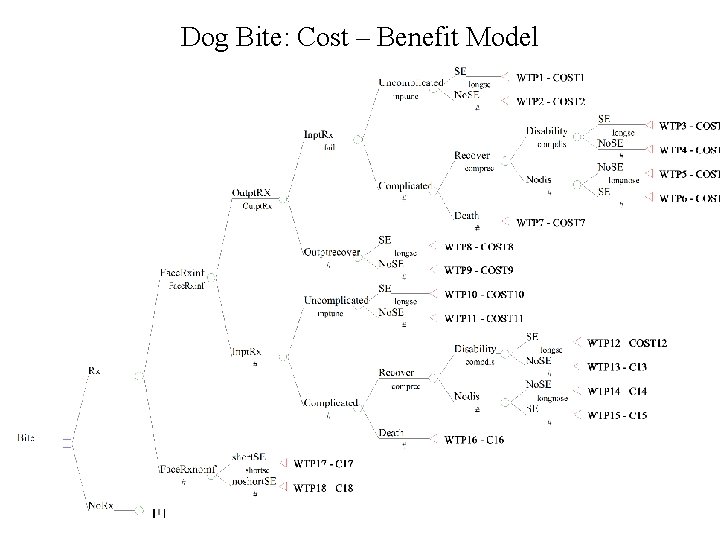
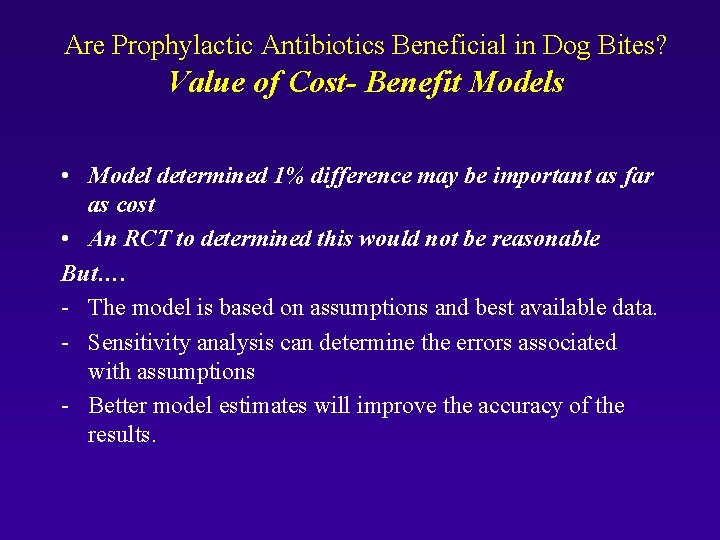
Are Prophylactic Antibiotics Beneficial in Dog Bites? Value of Cost- Benefit Models The models done ahead of a trial can; 1) Clearly define important outcomes to measure 2) Help determine MCID for sample size 3) Sometimes provide the answer

Dog Bite: Cost – Benefit Model

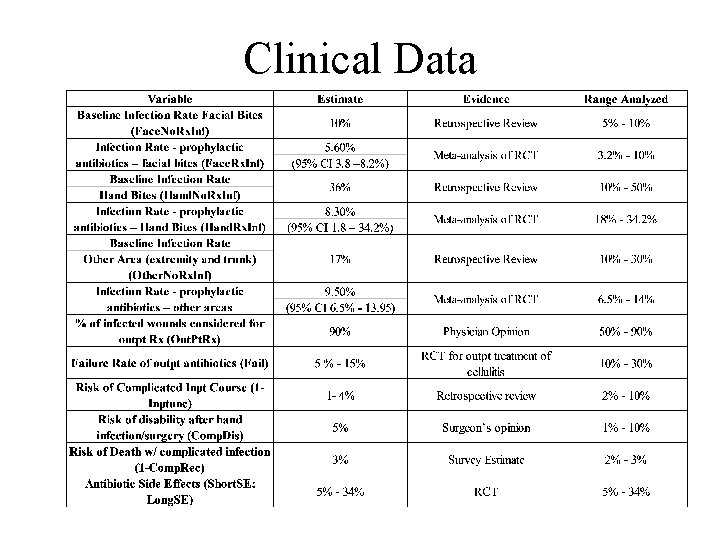
Clinical Data

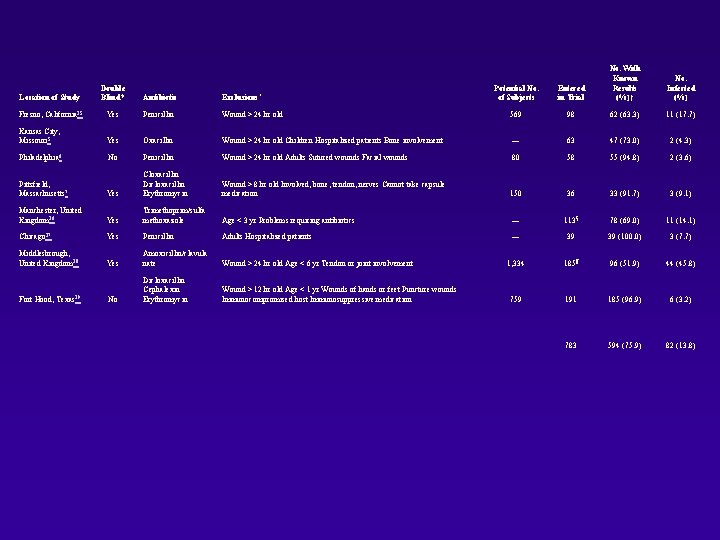
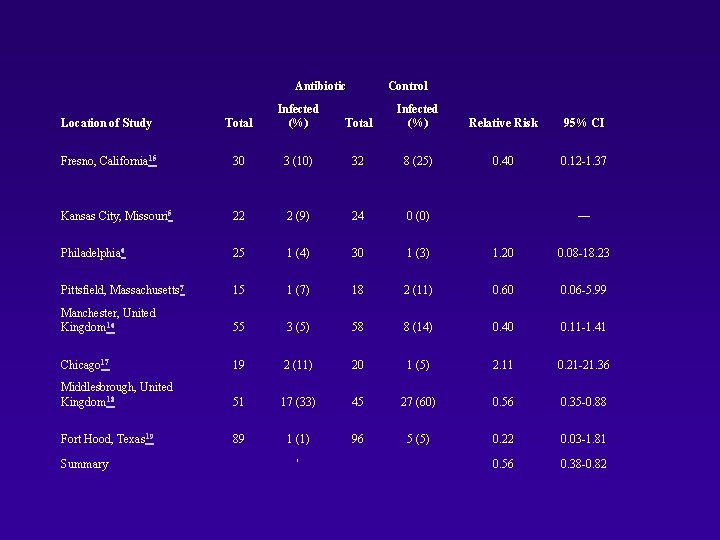
Potential No. of Subjects Entered in Trial No. With Known Results (%)† Wound > 24 hr old 569 98 62 (63. 3) 11 (17. 7) Oxacillin Wound > 24 hr old Children Hospitalized patients Bone involvement — 63 47 (73. 0) 2 (4. 3) No Penicillin Wound > 24 hr old Adults Sutured wounds Facial wounds 80 58 55 (94. 8) 2 (3. 6) Pittsfield, Massachusetts 7 Yes Cloxacillin Dicloxacillin Erythromycin Wound > 8 hr old Involved, bone, tendon, nerves Cannot take capsule medication 150 36 33 (91. 7) 3 (9. 1) Manchester, United Kingdom 16 Yes Trimethoprim/sulfa methoxazole Age < 3 yr Problems requiring antibiotics — 113§ 78 (69. 0) 11 (14. 1) Chicago 17 Yes Penicillin Adults Hospitalized patients — 39 39 (100. 0) 3 (7. 7) Middlesbrough, United Kingdom 18 Yes Amoxicillin/clavula nate Wound > 24 hr old Age < 6 yr Tendon or joint involvement 1, 334 185¶ 96 (51. 9) 44 (45. 8) No Dicloxacillin Cephalexin Erythromycin Wound > 12 hr old Age < 1 yr Wounds of hands or feet Puncture wounds Immunocompromised host Immunosuppressive medication 759 191 185 (96. 9) 6 (3. 2) 783 594 (75. 9) 82 (13. 8) Location of Study Double Blind? Antibiotic Exclusions* Fresno, California 15 Yes Penicillin Kansas City, Missouri 5 Yes Philadelphia 6 Fort Hood, Texas 19 No. Infected (%)

Antibiotic Control Location of Study Total Infected (%) Relative Risk 95% CI Fresno, California 15 30 3 (10) 32 8 (25) 0. 40 0. 12 -1. 37 Kansas City, Missouri 5 22 2 (9) 24 0 (0) — Philadelphia 6 25 1 (4) 30 1 (3) 1. 20 0. 08 -18. 23 Pittsfield, Massachusetts 7 15 1 (7) 18 2 (11) 0. 60 0. 06 -5. 99 Manchester, United Kingdom 16 55 3 (5) 58 8 (14) 0. 40 0. 11 -1. 41 Chicago 17 19 2 (11) 20 1 (5) 2. 11 0. 21 -21. 36 Middlesbrough, United Kingdom 18 51 17 (33) 45 27 (60) 0. 56 0. 35 -0. 88 Fort Hood, Texas 19 89 1 (1) 96 5 (5) 0. 22 0. 03 -1. 81 0. 56 0. 38 -0. 82 Summary '

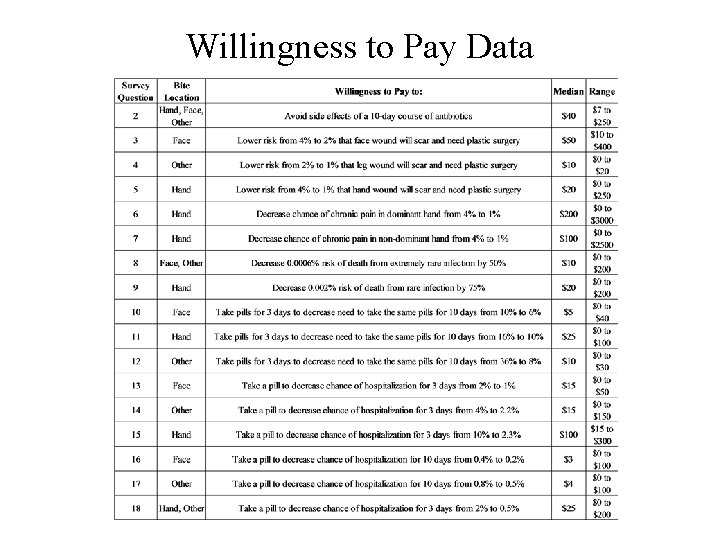
Willingness to Pay Data

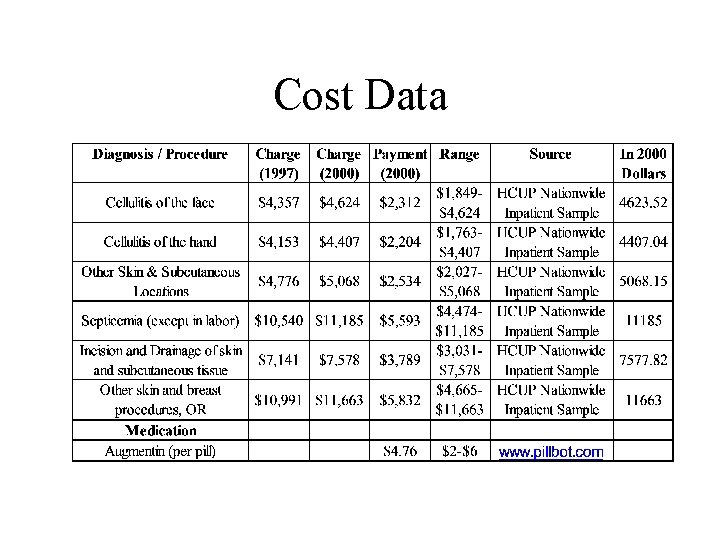
Cost Data



Are Prophylactic Antibiotics Beneficial in Dog Bites? Value of Cost- Benefit Models • Model determined 1% difference may be important as far as cost • An RCT to determined this would not be reasonable But…. - The model is based on assumptions and best available data. - Sensitivity analysis can determine the errors associated with assumptions - Better model estimates will improve the accuracy of the results.

Are Prophylactic Antibiotics Beneficial in Dog Bites • Funding – NIAMS • Design Cost Benefit Analysis with Clinical Trial Data • Start Aug 2003 UCSF add Stanford November 2004, Study will run through June 2006 • Patient randomized to 3 days of Augmentin or Placebo • Goal 100 – 125 patients outcomes (the largest trial) - 37 patients with complete F/U to date • Goal is to define and measure accurately all outcomes (infections, side effects, hospitalizations etc. ) in the model • Re-run the model and sensitivities to come up with the best recommendations.

Are Prophylactic Antibiotics Beneficial in Dog Bites? Outcomes • Infection - Defined as to whether the patient on followup was treated for an infection with antibiotics • Related physician/hospital: visits, admissions, treatments • Side effects - Self limited: patient self treated - Required physician visit/treatment

How Can I Help? • Expect a call when a dog bite comes in • Volunteers will do the enrolment if they are present, but will ask you some questions and will need physician help with attaining the consent • We will walk you through enrollment if the volunteers are not present • If you are too busy we will come in

Summary • Academic Emergency Medicine is growing • Research is an integral part • Division of Emergency Medicine at Stanford has made a commitment to the research program

Questions and Answers
 Jim quinn net worth
Jim quinn net worth Emergency nursing orientation
Emergency nursing orientation Whittington urgent care centre
Whittington urgent care centre Emergency care harrow
Emergency care harrow Sfgh emergency department
Sfgh emergency department Venerable edel quinn
Venerable edel quinn Ucf richard quinn
Ucf richard quinn Darwin quinn
Darwin quinn Marc quinn, self, 1991
Marc quinn, self, 1991 O mary conceived without sin
O mary conceived without sin John quinn beaumont
John quinn beaumont Ashlyn quinn
Ashlyn quinn Quinn versengő értékek modellje
Quinn versengő értékek modellje Instrumento ocai de cameron y quinn
Instrumento ocai de cameron y quinn Duane quinn
Duane quinn Feargal quinn
Feargal quinn Self – marc quinn, 1991
Self – marc quinn, 1991 Penny quinn
Penny quinn Quinn truss
Quinn truss Paulyn marrinan quinn
Paulyn marrinan quinn Unos region 5
Unos region 5 Jenny tsang-quinn
Jenny tsang-quinn Quinn prob
Quinn prob Socra clinical research
Socra clinical research Scientia clinical research
Scientia clinical research Research design in clinical psychology
Research design in clinical psychology Pi clinical research consultancy
Pi clinical research consultancy Alcoac
Alcoac Clinical research statistician
Clinical research statistician Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Kavi kenya
Kavi kenya Academic research organization
Academic research organization Translating research findings to clinical nursing practice
Translating research findings to clinical nursing practice Jasper clinical research
Jasper clinical research Asbmt clinical research training course
Asbmt clinical research training course Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Clinical research support services
Clinical research support services Importance of biostatistics
Importance of biostatistics Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Drcr.net
Drcr.net Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Questra clinical research
Questra clinical research Mrc clinical research training fellowship
Mrc clinical research training fellowship Foundations of clinical research applications to practice
Foundations of clinical research applications to practice Iwr clinical research
Iwr clinical research Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất













































































