CLINICAL RESEARCH AND THE NHS CLINICAL RESEARCH AND









- Slides: 10

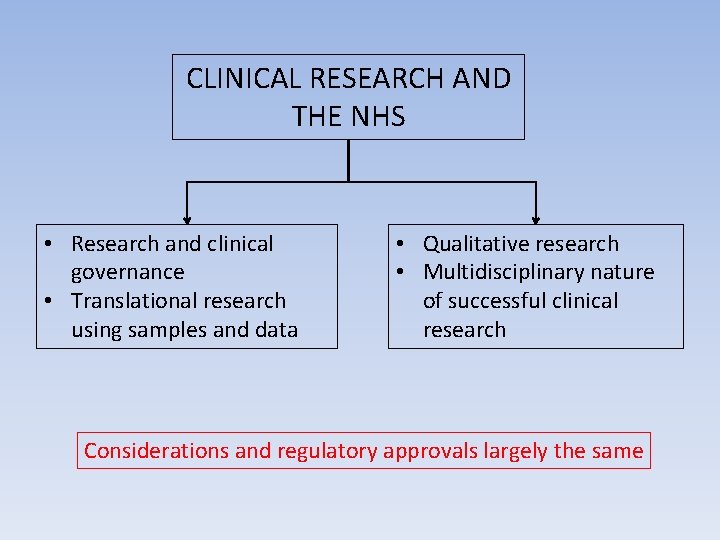
CLINICAL RESEARCH AND THE NHS

CLINICAL RESEARCH AND THE NHS • Research and clinical governance • Translational research using samples and data • Qualitative research • Multidisciplinary nature of successful clinical research Considerations and regulatory approvals largely the same

Who do you need on board? v Clinician/surgeon v Statistician v Pathologist v Clinical Trialist v Research Nurse

What approvals are required before you start? v Ethical approval v R&D approval (NHS permission and site-specific) v Clinical trials: MHRA, GTAC v Sponsorship

Ethical approval: Where do you apply? Non-NHS participants University Ethics Review Committee With or without sample collection/storage? NHS staff, premises, facilities University Ethics Review Committee NHS patients National Research Ethics Service (NRES) All types of research with or without sample collection

Ethical review: What will be considered? v v v Identification and recruitment Consent Scientific validity of the study Collection of samples; existing/prospective Access to personal/clinical data; anonymisation Storage and retention of personal data Funding Sponsorship Incidental findings and feedback What will happen to samples/data when study finished Vulnerable groups

R&D approval: What will be considered? v Principal Investigator v Identification and recruitment v Consent v Location v Whether procedures are additional to clinical pathway v Access to personal/clinical data; anonymisation v Storage and retention of personal data v Sponsorship v MONEY!

Clinical trials v Clinical trial authorisation from the MHRA v GTAC v Good Clinical Practice v Contractual agreements v Quality Management System v Adverse event reporting

Sponsor Under the Research Governance Framework …… Requirement for all research using NHS patients, staff and facilities The Sponsor is legally responsible for the management of the research v v v Investigators appropriate Funding Regulatory approvals Insurance Current legislation e. g. Human Tissue Act, DPA

Integrated Research Application Service (IRAS) Streamlining the research application process https: //www. myresearchproject. org. uk/ v v v Ethical approval NHS permission/R & D approval MHRA GTAC NOMS, MOJ